Bacitracin by Padagis US LLC BACITRACIN ointment
Bacitracin by
Drug Labeling and Warnings
Bacitracin by is a Prescription medication manufactured, distributed, or labeled by Padagis US LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- STERILE Rx Only
- DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
PRECAUTIONS:
Bacitracin ophthalmic ointment should not be used in deep-seated ocular infections or in those that are likely to become systemic. The prolonged use of antibiotic containing preparations may result in overgrowth of nonsusceptible organisms particularly fungi. If new infections develop during treatment appropriate antibiotic or chemotherapy should be instituted.
-
ADVERSE REACTIONS:
Bacitracin has such a low incidence of allergenicity that for all practical purposes side reactions are practically non-existent. However, if such reaction should occur, therapy should be discontinued.
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION:
The ointment should be applied directly into the conjunctival sac 1 to 3 times daily. In blepharitis all scales and crusts should be carefully removed and the ointment then spread uniformly over the lid margins. Patients should be instructed to take appropriate measures to avoid gross contamination of the ointment when applying the ointment directly to the infected eye.
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
-
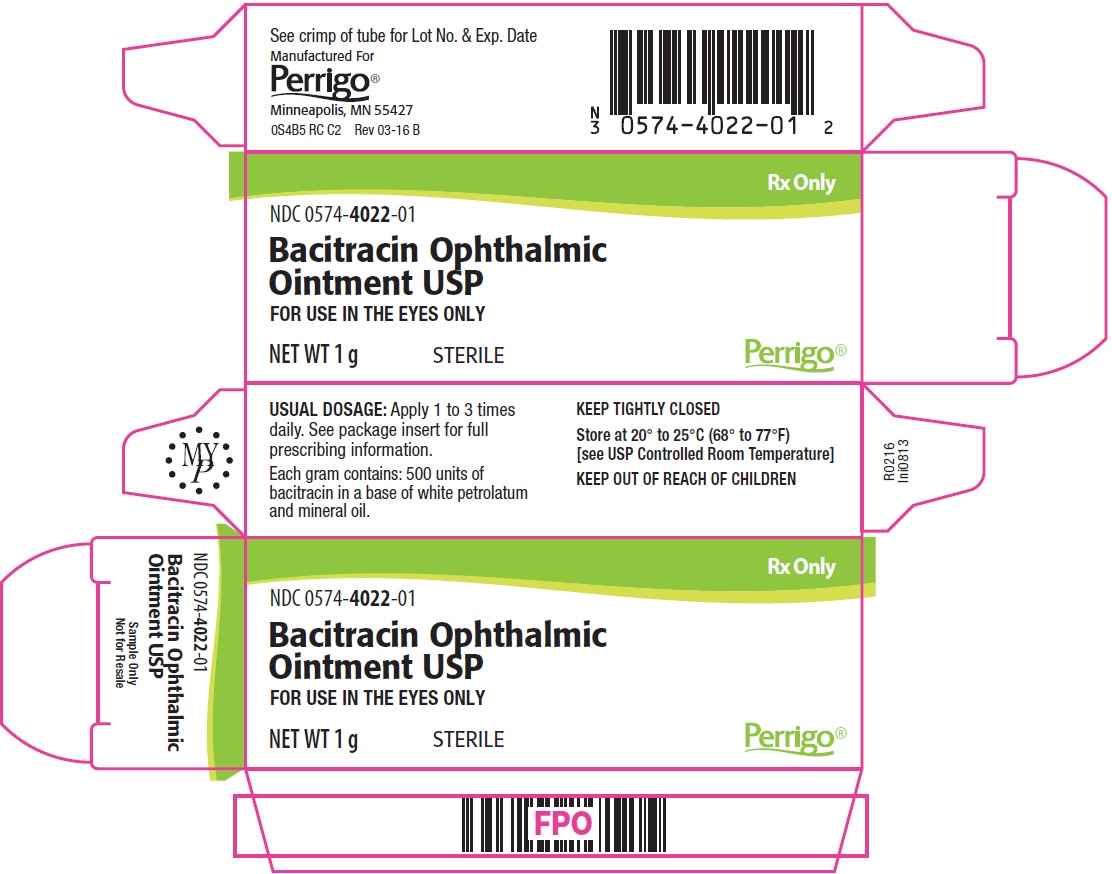
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1 g Carton (Sample Only)
Rx Only
NDC: 0574-4022-01
Bacitracin Ophthalmic Ointment USP
FOR USE IN THE EYES ONLY
NET WT 1 g
STERILE
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
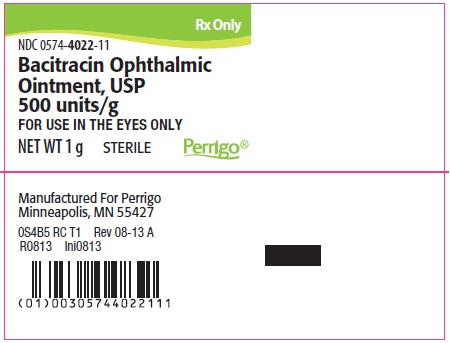
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1 g Label
Rx Only
NDC: 0574-4022-11
Bacitracin Ophthalmic Ointment, USP 500 units/g
FOR USE IN THE EYES ONLY
NET WT 1 g
STERILE
-
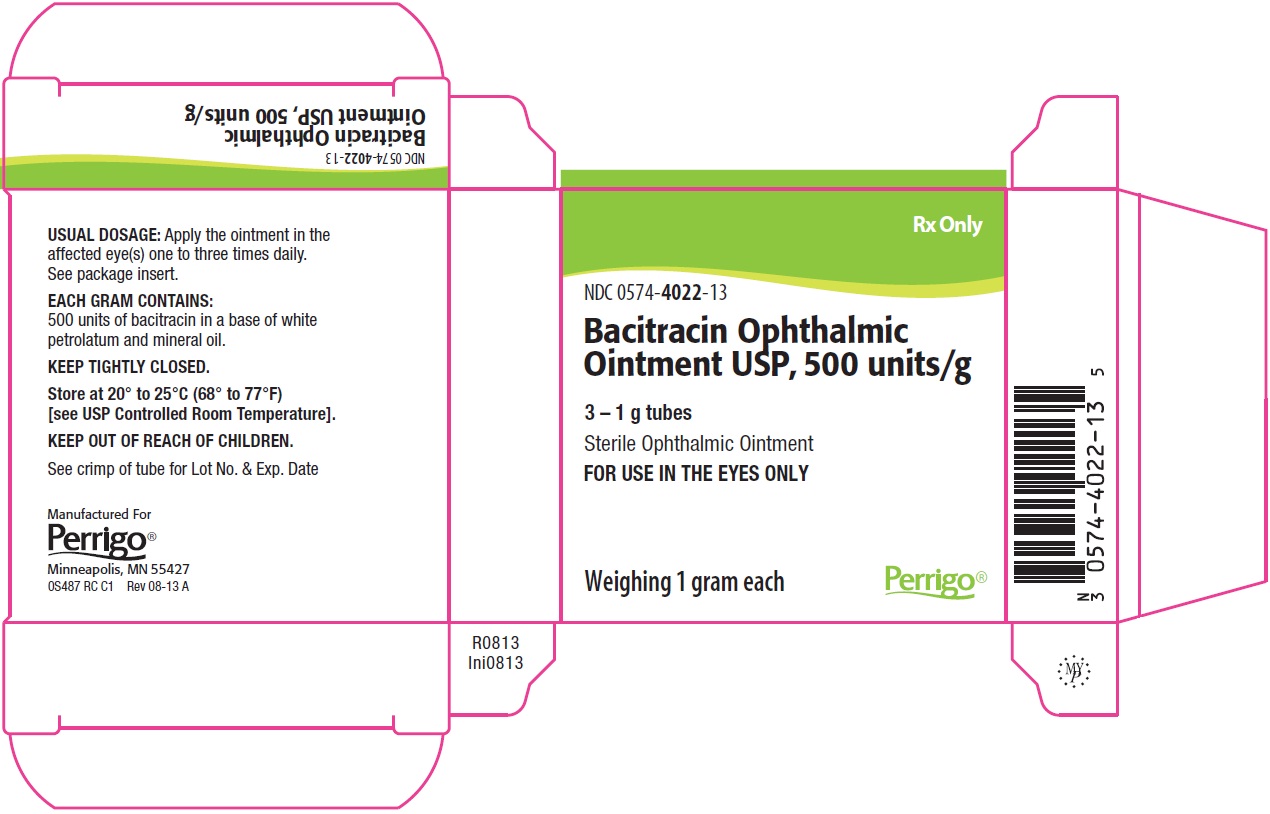
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 3 – 1 g Tube Carton
Rx Only
NDC: 0574-4022-13
Bacitracin Ophthalmic Ointment USP, 500 units/g
3 – 1 g tubes
Sterile Ophthalmic Ointment
FOR USE IN THE EYES ONLY
Weighing 1 gram each
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
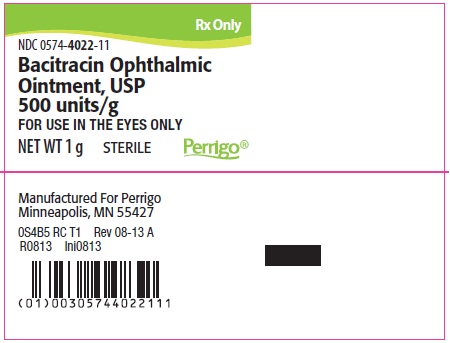
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1 g Label
Rx Only
NDC: 0574-4022-11
Bacitracin Ophthalmic Ointment, USP 500 units/g
FOR USE IN THE EYES ONLY
NET WT 1 g
STERILE
-
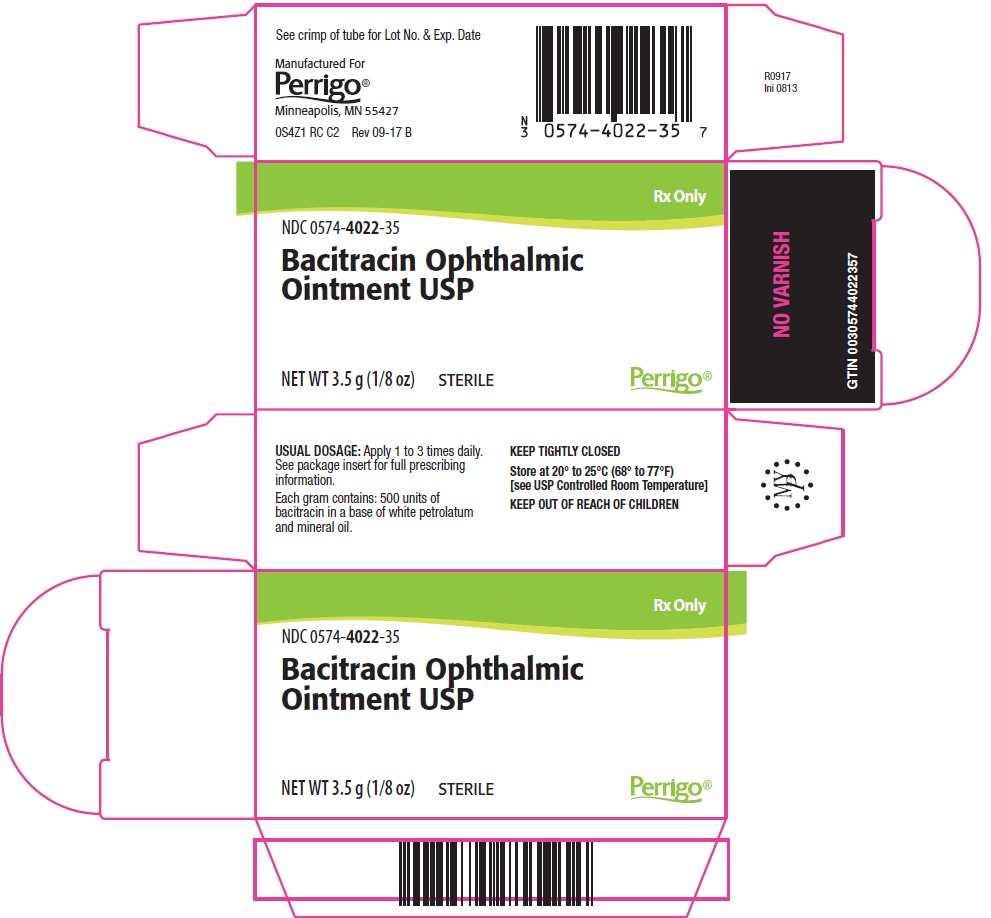
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 3.5 g Carton
Rx Only
NDC: 0574-4022-35
Bacitracin Ophthalmic Ointment USP
NET WT 3.5 g (1/8 oz)
STERILE
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 3.5 g Label
-
INGREDIENTS AND APPEARANCE
BACITRACIN
bacitracin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0574-4022 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN (UNII: 58H6RWO52I) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0574-4022-01 1 in 1 CARTON 03/10/2014 1 NDC: 0574-4022-11 1 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0574-4022-13 3 in 1 CARTON 03/10/2014 2 NDC: 0574-4022-11 1 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 0574-4022-35 1 in 1 CARTON 03/10/2014 3 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA061212 03/10/2014 Labeler - Paddock Laboratories, LLC (967694121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.