OLOPATADINE HYDROCHLORIDE solution/ drops
OLOPATADINE HYDROCHLORIDE by
Drug Labeling and Warnings
OLOPATADINE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by Somerset Therapeutics, LLC, Somerset Therapeutics Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
-
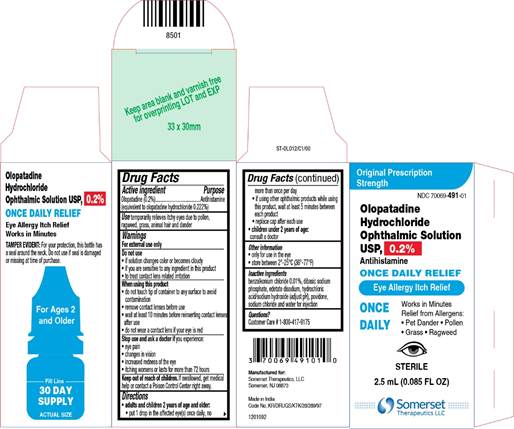
PRINCIPAL DISPLAY PANEL
NDC: 70069-491-01
Olopatadine Hydrochloride Ophthalmic Solution USP, 0.2%
Antihistamine
ONCE DAILY RELIEF
Sterile
2.5 mL (0.085 FL OZ)

Original Prescription Strength
NDC: 70069-491-01
Olopatadine Hydrochloride Ophthalmic Solution USP, 0.2%
Antihistamine
ONCE DAILY RELIEF
Eye Allergy Itch Relief
ONCE DAILY
Works in Minutes
Relief from Allergens:
Pet Dander
Pollen
Grass
Ragweed
Sterile
2.5 mL (0.085 FL OZ)
-
INGREDIENTS AND APPEARANCE
OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70069-491 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70069-491-01 2.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215006 12/20/2024 Labeler - Somerset Therapeutics, LLC (079947873) Registrant - Somerset Therapeutics, LLC (079947873) Establishment Name Address ID/FEI Business Operations Somerset Therapeutics Private Limited 677236695 ANALYSIS(70069-491) , LABEL(70069-491) , PACK(70069-491) , MANUFACTURE(70069-491)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.