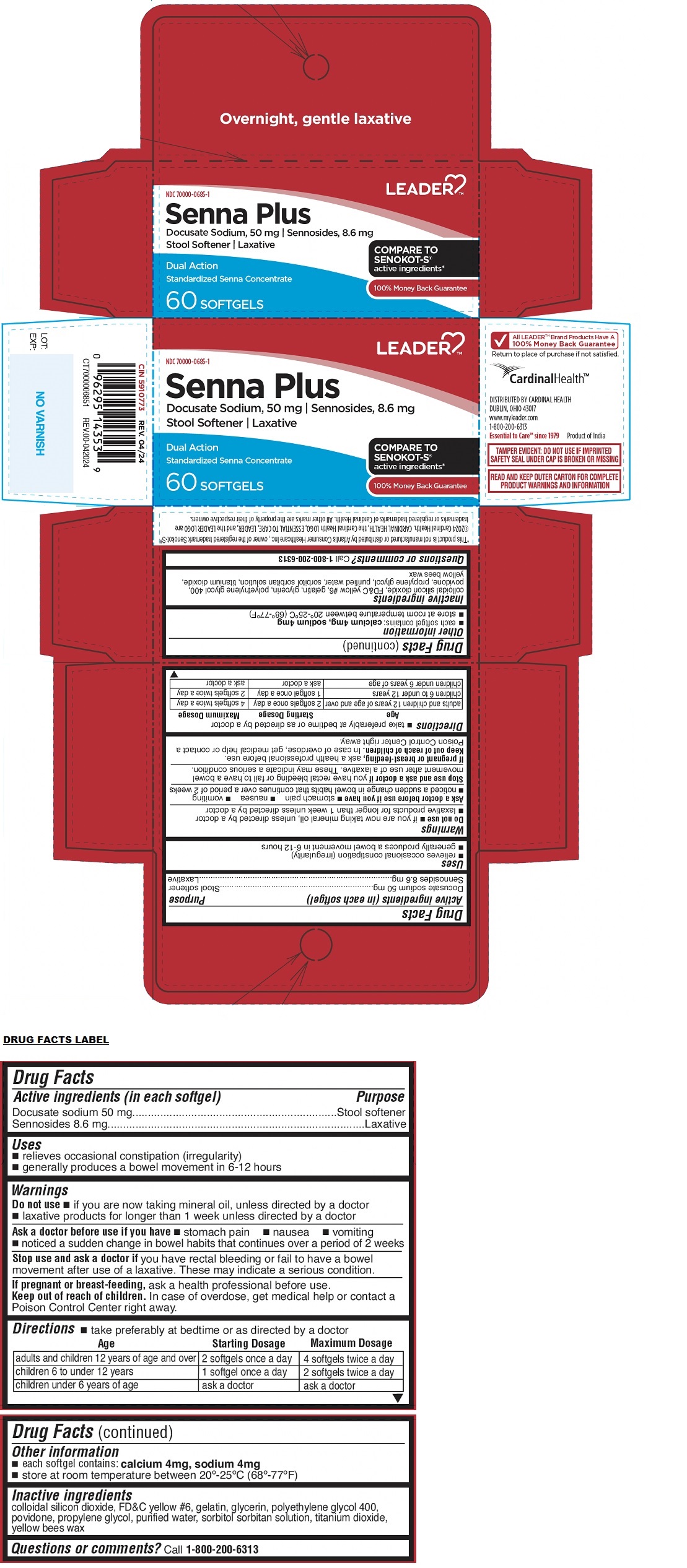
LEADER TMSenna Plus Stool Softener Laxative SOFTGELS
LEADER Senna Plus Stool Softener Laxative SOFTGEL by
Drug Labeling and Warnings
LEADER Senna Plus Stool Softener Laxative SOFTGEL by is a Otc medication manufactured, distributed, or labeled by Cardinal Health 110, LLC. DBA Leader. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEADER SENNA PLUS STOOL SOFTENER LAXATIVE SOFTGEL- docusate sodium, sennosides capsule, liquid filled
Cardinal Health 110, LLC. DBA Leader
----------
LEADER TMSenna Plus Stool Softener Laxative SOFTGELS
Uses
relieves occasional constipation (irregularity)
generally produces a bowel movement in 6-12 hours
Warnings
Do not use if you are now taking mineral oil, unless directed by a doctor laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have stomach pain nausea vomiting noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor ifyou have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
take preferably at bedtime or as directed by a doctor
| Age | Starting Dosage | Maximum Dosage |
| adults and children 12 years of age and over | 2 softgels once a day | 4 softgels twice a day |
| children 6 to under 12 years | 1 softgel once a day | 2 softgels twice a day |
| children under 6 years of age | ask a doctor | ask a doctor |
Other information
each softgel contains:
calcium 4 mg, sodium 4 mg
store at room temperature between 20°-25°C (68°-77°F)
Inactive ingredients
colloidal silicon dioxide, FD&C yellow #6, gelatin, glycerin, polyethylene glycol 400, povidone, propylene glycol, purified water, sorbitol sorbitan solution, titanium dioxide, yellow bees wax
COMPARE TO SENOKOT-S ® active ingredients*
Dual Action
Standardized Senna Concentrate
All LEADER™ Brand Products Have A
100% Money Back Guarantee
Return to place of purchase if not satisfied.
CardinalHealth™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com
1-800-200-6313
Essential to Care™ Since 1979
Product of India
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
READ AND KEEP OUTER CARTON FOR COMPLETE PRODUCT WARNINGS AND INFORMATION
Overnight, gentle laxative
*This product is not manufactured or distributed by Atlantis Consumer Healthcare Inc., owner of the registered trademark Senokot-S ®
©2024 Cardinal Health. CARDINAL HEALTH, the Cardinal Health LOGO, ESSENTIAL TO CARE, LEADER, and the LEADER LOGO are trademarks or registered trademarks of Cardinal Health. All other marks are the property of their respective owners.
| LEADER SENNA PLUS STOOL SOFTENER LAXATIVE SOFTGEL
docusate sodium, sennosides capsule, liquid filled |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Cardinal Health 110, LLC. DBA Leader (063997360) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOFTGEL HEALTHCARE PRIVATE LIMITED | 675584180 | manufacture(70000-0685) | |