HEM RID- hemorrhoidal suppositiories suppository
Hem Rid by
Drug Labeling and Warnings
Hem Rid by is a Otc medication manufactured, distributed, or labeled by Belligadria Sollutions LLC, Regulatory Matters Consulting. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- INACTIVE INGREDIENT
- WARNINGS
- INDICATIONS & USAGE
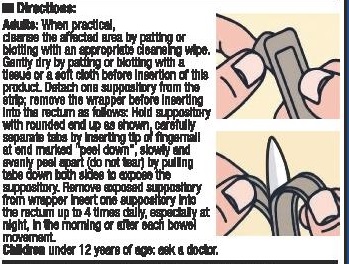
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEM RID
hemorrhoidal suppositiories suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71430-200 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCOA BUTTER (UNII: 512OYT1CRR) (COCOA BUTTER - UNII:512OYT1CRR) COCOA BUTTER 88.44 g in 100 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 0.25 g in 100 g Inactive Ingredients Ingredient Name Strength MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71430-200-12 12 in 1 BOX 07/01/2018 1 1 g in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 07/01/2018 Labeler - Belligadria Sollutions LLC (048891482) Registrant - Regulatory Matters Consulting (080711165)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.