icosapent ethyl by Softgel Healthcare Private Limited ICOSAPENT ETHYL capsule
icosapent ethyl by
Drug Labeling and Warnings
icosapent ethyl by is a Prescription medication manufactured, distributed, or labeled by Softgel Healthcare Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED
-
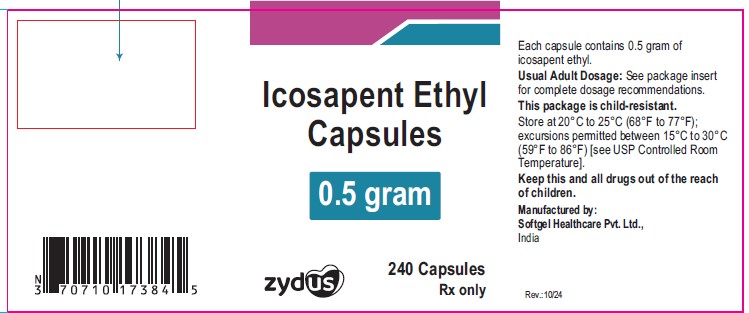
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 35916-1738-1
Icosapent Ethyl Capsules
0.5 gram
240 capsules
Rx only
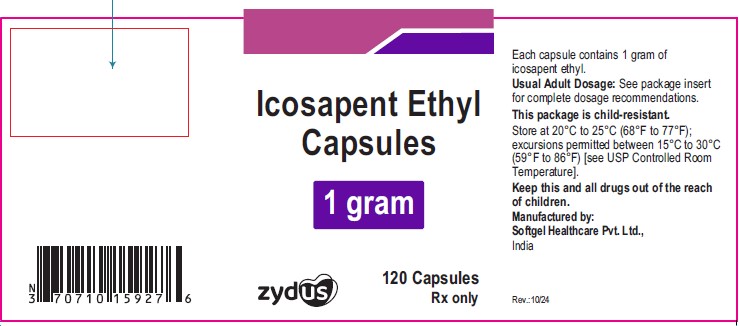
NDC: 35916-1592-1
Icosapent Ethyl Capsules
1 gram
240 capsules
Rx only
-
INGREDIENTS AND APPEARANCE
ICOSAPENT ETHYL
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 35916-1738 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICOSAPENT ETHYL (UNII: 6GC8A4PAYH) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT ETHYL 0.5 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE (transparent) Score no score Shape OVAL Size 14mm Flavor Imprint Code 1738 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 35916-1738-1 240 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217656 03/01/2025 ICOSAPENT ETHYL
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 35916-1592 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICOSAPENT ETHYL (UNII: 6GC8A4PAYH) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT ETHYL 1 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE (transparent) Score no score Shape OVAL (oblong) Size 24mm Flavor Imprint Code 1592 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 35916-1592-1 120 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217656 03/01/2025 Labeler - Softgel Healthcare Private Limited (675584180) Establishment Name Address ID/FEI Business Operations Softgel Healthcare Private Limited 675584180 ANALYSIS(35916-1738, 35916-1592) , LABEL(35916-1738, 35916-1592) , MANUFACTURE(35916-1738, 35916-1592) , PACK(35916-1738, 35916-1592)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.