TONMYA- cyclobenzaprine hydrochloride tablet, orally disintegrating
TONMYA by
Drug Labeling and Warnings
TONMYA by is a Prescription medication manufactured, distributed, or labeled by Tonix Medicines, Inc., Tonix Pharmaceuticals, Inc., Almac Pharma Services Limited, Patheon Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TONMYA safely and effectively. See full prescribing information for TONMYA.
TONMYA™ (cyclobenzaprine hydrochloride sublingual tablets)
Initial U.S. Approval: 1977INDICATIONS AND USAGE
TONMYA is indicated for the treatment of fibromyalgia in adults (1).
DOSAGE AND ADMINISTRATION
- Recommended dosage of TONMYA is 5.6 mg administered sublingually once daily at bedtime (2.1):
- Starting dose: Days 1 to 14, administer 2.8 mg (1 sublingual tablet) once daily at bedtime.
- Target dose: Days 15 and thereafter, administer 5.6 mg (2 sublingual tablets) once daily at bedtime.
- Maximum recommended dosage: 5.6 mg once daily.
- Ensure mouth is moist with sips of water before sublingual administration (2.4, 5.6, 6.1)
- Do not swallow whole, cut, crush, or chew (2.4)
- Geriatric patients: Recommended dosage is 2.8 mg administered sublingually once daily at bedtime (2.2, 8.5)
- Hepatic impairment (HI): Recommended dosage in patients with mild HI is 2.8 mg administered sublingually once daily at bedtime. Use not recommended in patients with moderate HI or severe HI (2.3, 8.6).
- See important administration instructions in the Full Prescribing Information (2.4, 2.5, 2.6).
DOSAGE FORMS AND STRENGTHS
Sublingual tablets: 2.8 mg of cyclobenzaprine hydrochloride (3).
CONTRAINDICATIONS
- Hypersensitivity to cyclobenzaprine or any inactive ingredient in TONMYA (4)
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation (4)
- During acute recovery phase of myocardial infarction and in patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure (4)
- Hyperthyroidism (4)
WARNINGS AND PRECAUTIONS
- Embryofetal Toxicity: Based on animal data, TONMYA may cause neural tube defects when used two weeks prior to conception and through the first trimester of pregnancy. Advise females of reproductive potential of the potential risk and to use effective contraception during treatment and for two weeks after the final dose. (5.1, 8.1, 8.3)
- Serotonin Syndrome: Potentially life-threatening serotonin syndrome has been reported with cyclobenzaprine when used in combination with other drugs. Immediately discontinue concomitant treatment with TONMYA and a serotonergic agent if serotonin syndrome symptoms occur and initiate supportive symptomatic treatment. If concomitant treatment with TONMYA and other serotonergic drugs is clinically warranted, careful observation is advised, particularly during treatment initiation or dosage increases. (5.2)
- Tricyclic Antidepressant-like Adverse Reactions: TCAs have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke. TCAs lower the seizure threshold, and are associated with serious CNS reactions. If clinically significant CNS symptoms develop, consider discontinuation of TONMYA. (5.3)
- Atropine-like Adverse Reactions: Use with caution in patients with a history of urinary retention, angle-closure glaucoma, increased intraocular pressure and in patients taking anticholinergic medications. (5.4)
- CNS Depression and Risk of Operating a Motor Vehicle or Hazardous Machinery: TONMYA monotherapy may cause CNS depression. Advise patients not to operate a motor vehicle or other dangerous machinery until they are reasonably certain that TONMYA therapy will not adversely affect their ability to engage in such activities. (5.5)
- Oral Mucosal Adverse Reactions: In clinical studies, oral mucosal adverse reactions occurred more frequently in TONMYA-treated patients compared to placebo-treated patients. Advise patients to moisten the mouth with sips of water before administration of TONMYA to reduce the risk of oral sensory changes (hypoesthesia). Consider discontinuation of TONMYA if severe reactions occur. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2% and at a higher incidence in TONMYA-treated patients compared to placebo-treated patients): oral hypoesthesia, oral discomfort, abnormal product taste, somnolence, oral paresthesia, oral pain, fatigue, dry mouth, and aphthous ulcer (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Tonix Medicines, Inc. at 1-888-869-7633 (1-888-TNXPMED) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- MAO Inhibitors: Life-threatening interactions may occur (4, 7)
- Other serotonergic Drugs: Serotonin syndrome has been reported (5.2, 7)
- CNS Depressants: CNS depressant effects of alcohol, barbiturates, and other CNS depressants may be enhanced (5.5, 7)
- Tramadol: Seizure risk may be enhanced (7)
- Guanethidine: Antihypertensive effect may be blocked (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2025
- Recommended dosage of TONMYA is 5.6 mg administered sublingually once daily at bedtime (2.1):
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Dosage in Geriatric Patients
2.3 Recommended Dosage in Patients with Hepatic Impairment
2.4 Administration Instructions
2.5 Recommendations Regarding Missed Dose(s)
2.6 Pregnancy Testing Prior to Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryofetal Toxicity
5.2 Serotonin Syndrome
5.3 Tricyclic Antidepressant-like Adverse Reactions
5.4 Atropine-like Adverse Reactions
5.5 CNS Depression and Risk of Operating a Motor Vehicle or Hazardous Machinery
5.6 Oral Mucosal Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of TONMYA is 5.6 mg administered sublingually once daily at bedtime:
- Starting dose: Days 1 to 14, administer 2.8 mg (1 sublingual tablet) once daily at bedtime.
- Target dose: Days 15 and thereafter, administer 5.6 mg (2 sublingual tablets) once daily at bedtime.
- Maximum recommended dosage: 5.6 mg once daily.
2.2 Recommended Dosage in Geriatric Patients
The recommended TONMYA dosage―and the maximum recommended dosage―in geriatric patients is 2.8 mg administered sublingually once daily at bedtime [see Use in Specific Populations (8.5)].
2.3 Recommended Dosage in Patients with Hepatic Impairment
The recommended TONMYA dosage―and the maximum recommended dosage―in patients with mild hepatic impairment is 2.8 mg administered sublingually once daily at bedtime. TONMYA is not recommended in patients with moderate or severe hepatic impairment [see Use in Specific Populations (8.6)].
2.4 Administration Instructions
TONMYA is only for sublingual use.
- Administer after brushing teeth and finishing other oral care and ensure a moist mouth/sublingual area by drinking a few sips of water prior to administration.
- Place the sublingual tablet(s) under the tongue until dissolved.
- Do not swallow whole, cut, crush, or chew.
- Avoid eating or drinking for at least 15 minutes after the sublingual tablet(s) has/have completely dissolved at bedtime and preferably avoid any hot, cold, or acidic beverages until the morning.
- Avoid talking for at least 5 minutes after administration.
2.5 Recommendations Regarding Missed Dose(s)
If you missed a TONMYA bedtime dose, take TONMYA the next evening. Do not take a missed dose during the day.
2.6 Pregnancy Testing Prior to Administration
Pregnancy testing is recommended in females of reproductive potential prior to initiating treatment with TONMYA [see Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.3)]
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Hypersensitivity to cyclobenzaprine or any inactive ingredient in TONMYA. Hypersensitivity reactions may manifest as an anaphylactic reaction, urticaria, facial and/or tongue swelling, or pruritus. Discontinue TONMYA if a hypersensitivity reaction is suspected.
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after discontinuation of a MAO inhibitor. Hyperpyretic crisis seizures and deaths have occurred in patients who received cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitors drugs.
- During the acute recovery phase of myocardial infarction, and in patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
- Hyperthyroidism.
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryofetal Toxicity
Based on data from animal reproduction studies, TONMYA may cause an increased risk of neural tube defects when administered to a pregnant female two weeks prior to conception and during the first trimester of pregnancy. Neural tube defects (splayed vertebral arches and spina bifida occulta) were observed in a rabbit embryofetal development study at the highest maternal dose tested, in the absence of maternal toxicity. Because neural tube development occurs early in pregnancy, often before pregnancy is recognized, advise females of reproductive potential of the potential risk to the fetus and avoid use of TONMYA two weeks prior to conception and through the first trimester of pregnancy.
Perform a pregnancy test prior to initiation of treatment with TONMYA to exclude use of TONMYA during the first trimester of pregnancy.
Advise females of reproductive potential to use effective contraception during TONMYA treatment and for two weeks after the final dose [see Use in Specific Populations (8.1, 8.3)].
5.2 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with cyclobenzaprine when used in combination with other drugs, such as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. The concomitant use of TONMYA with MAO inhibitors is contraindicated [see Contraindications (4)].
Serotonin syndrome symptoms may include mental status changes (e.g., confusion, agitation, hallucinations), autonomic instability (e.g., diaphoresis, tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., tremor, ataxia, hyperreflexia, clonus, muscle rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Treatment with TONMYA and any concomitant serotonergic agents should be discontinued immediately if serotonin syndrome symptoms occur and supportive symptomatic treatment should be initiated. If concomitant treatment with TONMYA and other serotonergic drugs is clinically warranted, careful observation is advised, particularly during treatment initiation or dosage increases.
5.3 Tricyclic Antidepressant-like Adverse Reactions
Cyclobenzaprine is structurally related to tricyclic antidepressants (TCAs).
TCAs have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke [see Contraindications (4)].
Some of the more TCA-associated serious central nervous system (CNS) reactions have occurred in short-term studies of oral cyclobenzaprine. If clinically significant CNS symptoms develop, consider discontinuation of TONMYA.
Caution should be used when TCAs are given to patients with a history of seizure disorder, because TCAs may lower the seizure threshold. Patients with a history of seizures should be monitored during TCA use to identify recurrence of seizures or increase in frequency of seizures.
5.4 Atropine-like Adverse Reactions
Because of its atropine-like action, TONMYA should be used with caution in patients with a history of urinary retention, angle-closure glaucoma, increased intraocular pressure, and in patients taking anticholinergic drugs.
5.5 CNS Depression and Risk of Operating a Motor Vehicle or Hazardous Machinery
TONMYA monotherapy may cause CNS depression and concomitant use of TONMYA with alcohol, barbiturates, or other CNS depressants may increase the risk of CNS depression [see Drug Interactions (7)].
Symptoms of CNS depression include somnolence. Advise patients not to operate a motor vehicle or dangerous machinery until they are reasonably certain that TONMYA therapy will not adversely affect their ability to engage in such activities.
5.6 Oral Mucosal Adverse Reactions
Oral mucosal adverse reactions, including sensory changes (e.g., numbness, tingling), discomfort, pain, irritation, inflammation, and lesions, occurred more frequently in patients treated with TONMYA compared to placebo (43% vs. 8%) [see Adverse Reactions (6.1)]. Reactions typically occurred within minutes of administration and most resolved within 60 minutes.
Five patients experienced severe oral mucosal adverse reactions, including sensory changes (paresthesia, hypoesthesia), inflammation (glossitis), oral pain, and dry mouth. Most severe oral mucosal adverse reactions resolved within days after TONMYA was discontinued and no treatment was required.
Advise patients to moisten the mouth with sips of water before administration of TONMYA to reduce the risk of oral sensory changes (hypoesthesia). Advise patients to report severe oral mucosal adverse reactions to their healthcare provider. Consider discontinuation of TONMYA if severe reactions occur.
-
6 ADVERSE REACTIONS
The following clinically significant reactions are described in greater detail, in other sections of this labeling:
- Embryofetal Toxicity [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Tricyclic Antidepressant-like Adverse Reactions [see Warnings and Precautions (5.3)]
- Atropine-like Adverse Reactions [see Warnings and Precautions (5.4)]
- CNS Depression [see Warnings and Precautions (5.5)]
- Oral Mucosal Adverse Reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of sublingual TONMYA (14 days of 2.8 mg once daily and then 5.6 mg once daily thereafter) is supported by three double-blind, placebo-controlled clinical trials (Trials 1, 2, and 3) in adult patients with fibromyalgia [see Clinical Studies (14)]. A total of 1,182 patients completed at least 14 weeks of daily treatment, including 580 TONMYA-treated patients (14 days of 2.8 mg once daily and then 5.6 mg once daily thereafter) and 602 placebo-treated patients.
Table 1 summarizes the most common adverse reactions in Trials 1, 2, and 3 (≥2% of TONMYA-treated patients and at a higher incidence in TONMYA-treated patients compared to placebo-treated patients).
Table 1: Adverse Reactions Reported in ≥2% of TONMYA-Treated Patients and a Higher Incidence than Placebo-Treated Patients in Adult Patients with Fibromyalgia (Trials 1, 2, and 3) Adverse Reactions Placebo
(N = 739)TONMYA
(N = 735)- * Oral hypoesthesia includes hypoesthesia and teeth hypoesthesia
- † Oral discomfort includes tongue discomfort
- ‡ Somnolence includes hypersomnia, lethargy, and sedation
- § Oral paresthesia includes paresthesia and teeth hyperesthesia
- ¶ Oral pain includes glossodynia
- # Fatigue includes asthenia and lethargy
- Þ Dry mouth includes dry throat
Oral hypoesthesia* 0.7% 23% Oral discomfort† 0.7% 9% Abnormal product taste 0.7% 9% Somnolence‡ 2% 6% Oral paresthesia§ 0.4% 6% Oral pain¶ 1% 5% Fatigue# 2% 4% Dry mouthÞ 2% 3% Aphthous ulcer 0.5% 2% Oral Mucosal Adverse Reactions in Trials 1, 2, and 3
In Trials 1, 2, and 3, 43% of TONMYA-treated patients compared to 8% of placebo-treated patients experienced at least 1 treatment-emergent oral mucosal adverse reaction. The most common oral mucosal adverse reactions included oral hypoesthesia, abnormal product taste, oral paresthesia, tongue discomfort, oral discomfort, glossodynia, oral pain, and aphthous ulcer. The majority (82%) of oral mucosal adverse reactions began within minutes of dosing, and of those, 88% occurred after nearly every dose. Almost two-thirds lasted less than 60 minutes. Of the approximately one-third that lasted longer than 60 minutes, 63% were present the next morning.
Five patients (0.7% of TONMYA-treated patients) experienced severe oral mucosal adverse reactions, including paresthesia, glossitis, hypoesthesia, oral pain, and dry mouth. Most reactions resolved within days after TONMYA was discontinued. Oral mucosal adverse reactions leading to discontinuation occurred more frequently in TONMYA-treated patients compared to placebo-treated patients (4.5% vs. 0.5%).
Adverse Reactions from Other Trials
In an open-label, long-term 40 to 52-week safety trial (Trial 4) in an unapproved population of patients previously exposed to 5.6 mg TONMYA once daily (maximum recommended dosage) or placebo, 56 patients were treated with 5.6 mg of TONMYA for at least 1 year. The most common adverse reactions in the TONMYA-treated patients (>5%) were oral hypoesthesia (45%), somnolence (18%), abnormal product taste (7%), and paresthesia oral (7%).
In an open-label, long-term 52 week safety trial of adult patients with fibromyalgia previously been exposed to 2.8 mg TONMYA once daily (one half the recommended dosage [see Dosage and Administration (2.1)]) or placebo (Trial 5), 97 patients were treated for at least 1 year with 2.8 mg of TONMYA once daily. The most common adverse reactions (>5%) in the TONMYA-treated patients were hypoesthesia oral (15%), fatigue (7%), sinusitis (7%), and abnormal product taste (6%).
6.2 Postmarketing Experience
The following adverse reactions have been reported in clinical studies or postmarketing experience with cyclobenzaprine immediate-release (IR) products, cyclobenzaprine extended-release (ER) products, or TCAs. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In a postmarketing surveillance program of cyclobenzaprine IR products, the adverse reactions reported most frequently were drowsiness, dry mouth, and dizziness, and adverse reactions reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion.
The following adverse reactions have been reported in postmarketing experience with cyclobenzaprine ER products or cyclobenzaprine IR products, in clinical studies of cyclobenzaprine IR products (incidence <1%), or in postmarketing experience with other TCAs:
Body as a Whole: Syncope; malaise; chest pain; edema.
Cardiovascular: Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension; hypertension; myocardial infarction; heart block; stroke.
Digestive: Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice, and cholestasis; paralytic ileus, tongue discoloration; stomatitis; parotid swelling.
Endocrine: Inappropriate ADH syndrome.
Hematologic and Lymphatic: Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia.
Hypersensitivity: Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash.
Metabolic, Nutritional, and Immune: Elevation and lowering of blood sugar levels; weight gain or loss.
Musculoskeletal: Local weakness; myalgia.
Nervous System and Psychiatric: Seizures, ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis, abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia; serotonin syndrome; neuroleptic malignant syndrome; decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell's palsy; alteration in EEG patterns; extrapyramidal symptoms.
Respiratory: Dyspnea.
Skin: Sweating; photosensitization; alopecia.
Special Senses: Ageusia; tinnitus.
Urogenital: Urinary frequency and/or retention; impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea.
-
7 DRUG INTERACTIONS
Based on its structural similarity to TCAs, concomitant use of TONMYA with:
- MAO inhibitors may be life-threatening [see Contraindications (4)],
- Alcohol, barbiturates, and other CNS depressants may increase the risk of adverse reactions associated with these drugs,
- Tramadol may increase the seizure risk,
- Guanethidine or other similar acting drugs may block the antihypertensive action of these drugs.
Postmarketing cases of serotonin syndrome have been reported with the concomitant use of oral cyclobenzaprine and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors [see Warnings and Precautions (5.2)].
- The concomitant use of TONMYA with MAO inhibitors is contraindicated. If serotonin syndrome symptoms occur with the use of other serotonergic drugs, immediately discontinue TONMYA.
- If concomitant treatment with TONMYA and other serotonergic drugs (besides MAO inhibitors) is clinically warranted, careful observation is advised, particularly during dosage increases.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data, TONMYA may cause fetal harm when administered to a pregnant woman. In rabbits, an increased incidence of neural tube defects (splayed vertebral arches and spina bifida occulta) was observed when pregnant rabbits were treated with oral cyclobenzaprine during embryogenesis with 30 mg/kg/day (approximately 0.2 times the maximum recommended human dose (MRHD) of TONMYA), in the absence of maternal toxicity. In rats, decreased pup body weight and survival were noted at a cyclobenzaprine dose of ≥10 mg/kg/day (approximately ≥0.8 times the MRHD of TONMYA), when administered orally during pregnancy and lactation (see Data). The limited amount of available observational data on oral cyclobenzaprine use in pregnancy is of insufficient quality to inform a TONMYA-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Advise pregnant women about the potential risk to the fetus with maternal exposure to TONMYA and to avoid use of TONMYA two weeks prior to conception and through the first trimester of pregnancy.
The background risk of major birth defects and miscarriage for pregnant women with fibromyalgia is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report Pregnancies to the Tonix Medicines, Inc., Adverse Event reporting line at 1-888-869-7633 (1-888-TNXPMED).
Animal Data:
Oral administration of cyclobenzaprine during organogenesis to rabbits at the highest maternal dose of 30 mg/kg/day (approximately 0.2 times the MRHD of 5.6 mg/day of TONMYA on an AUC basis) increased the incidence of neural tube defects (splayed vertebral arches and spina bifida occulta) in the absence of maternal toxicity. The no effect level for embryo-fetal development in rabbits was 10 mg/kg/day (approximately 0.05 times the MRHD of TONMYA on an AUC basis).
In another study, no adverse embryofetal effects were reported following oral administration of cyclobenzaprine during organogenesis to rabbits at maternal doses up to 20 mg/kg/day (approximately 0.1 times the MRHD of TONMYA on an estimated AUC basis).
No adverse embryofetal effects were reported following oral administration of cyclobenzaprine during organogenesis to rats at doses up to 25 mg/kg/day (approximately 9.2 times the MRHD of TONMYA, on an AUC basis). Maternal toxicity characterized by decreased body weight gain was observed in rats at this dose of 25 mg/kg/day.
No adverse embryofetal effects were reported following oral administration of cyclobenzaprine during organogenesis to mice at maternal doses up to 20 mg/kg/day (approximately 17 times the MRHD of TONMYA on a mg/m2 basis). Maternal toxicity characterized by decreased body weight gain was observed at the highest tested dose of 20 mg/kg/day.
Decreased pup body weight and survival were reported in a prenatal and postnatal study where pregnant rats were treated orally with cyclobenzaprine during pregnancy and lactation with maternal doses of 10 and 20 mg/kg/day (approximately 0.8 and 1.7 times the MRHD of TONMYA on an estimated AUC basis). Maternal toxicity, characterized by a decreased body weight gain, was observed only at the highest tested dose of 20 mg/kg/day.
In another prenatal and postnatal study where pregnant rats were treated orally with cyclobenzaprine during pregnancy and lactation with maternal doses up to 10 mg/kg/day (approximately 0.8 times the MRHD of TONMYA on an AUC basis) no adverse effects were reported in maternal animals and offspring.
8.2 Lactation
Risk Summary
A small number of published cases report the transfer of cyclobenzaprine into human milk in low amounts, but these data cannot be confirmed. There are no data on the effects of cyclobenzaprine on a breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TONMYA and any potential adverse effects on the breastfed child from TONMYA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Based on animal data, TONMYA may cause neural tube defects at clinically relevant doses. Females of reproductive potential should avoid use of TONMYA two weeks prior to conception through the first trimester of pregnancy [see Use in Specific Populations (8.1)].
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiation of treatment with TONMYA.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with TONMYA and for two weeks after the final dose.
8.4 Pediatric Use
The safety and effectiveness of TONMYA have not been established in pediatric patients.
8.5 Geriatric Use
Of the total number of TONMYA-treated patients in the clinical trials in adult patients with fibromyalgia (Trials 1, 2, and 3) [see Clinical Studies (14)], none were 65 years of age and older. Clinical trials of TONMYA did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
After administration of cyclobenzaprine extended release capsules, the plasma concentration and half-life of cyclobenzaprine were substantially increased in the subjects 65 years of age and older compared to those under 65 years of age [see Clinical Pharmacology (12.3)]. Given the higher cyclobenzaprine exposure in patients 65 years of age and older, the recommended TONMYA dosage in patients 65 years of age and older is 2.8 mg once daily at bedtime, lower than the recommended dosage in younger adult patients [see Dosage and Administration (2.2)].
8.6 Hepatic Impairment
The recommended dosage of TONMYA in patients with mild hepatic impairment (HI) (Child Pugh A) is 2.8 mg once daily at bedtime, lower than the recommended dosage in patients with normal hepatic function [see Dosage and Administration (2.2)]. The use of TONMYA is not recommended in patients with moderate HI (Child Pugh B) or severe HI (Child Pugh C).
Cyclobenzaprine exposure (AUC) was increased in patients with mild HI and moderate HI compared to subjects with normal hepatic function [see Clinical Pharmacology (12.3)], which may increase the risk of TONMYA-associated adverse reactions.
- 9 DRUG ABUSE AND DEPENDENCE
-
10 OVERDOSAGE
Overdose Signs, Symptoms, and Complications of Cyclobenzaprine Overdose
- The most common adverse reactions associated with cyclobenzaprine overdose are drowsiness and tachycardia.
- Less frequent overdose manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations.
- Rare but potentially critical overdose manifestations that have been reported are cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, cases of neuroleptic malignant syndrome and rhabdomyolysis, and death.
Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of cyclobenzaprine toxicity. Other potential cyclobenzaprine overdosage adverse reactions include any of the adverse reactions listed under Adverse Reactions (6).
Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose.
Treatment of Cyclobenzaprine Overdose
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
Signs and symptoms of toxicity may develop rapidly after cyclobenzaprine overdose; therefore, hospital monitoring is recommended as soon as possible. To reduce the risk of rare but potentially critical cyclobenzaprine overdose manifestations, obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway and establish an intravenous line. Recommend observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures.
Monitoring of plasma cyclobenzaprine levels should not guide overdose management of the patient. Dialysis is probably of no value because of low plasma concentrations of cyclobenzaprine.
Treatment of Cardiovascular Overdosage Complications: A maximal limb-lead QRS duration of 0.1 seconds may be the best indication of the severity of the cyclobenzaprine overdose.
Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed), should be instituted for patients with dysrhythmias and/or QRS widening. A pH >7.6 or a pCO2 <20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine or phenytoin. Type 1A and 1C antiarrhythmics (e.g., quinidine, disopyramide, and procainamide) are generally contraindicated in the setting of a cyclobenzaprine overdose.
Treatment of CNS Overdosage Complications: In patients with CNS depression associated with a cyclobenzaprine overdose, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines or, if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in close consultation with a poison center.
-
11 DESCRIPTION
Cyclobenzaprine hydrochloride, USP is a white to off-white crystalline tricyclic amine salt powder, 1-Propanamine, 3-(5H-dibenzo[a,d] cyclohepten-5-ylidene)-N,N-dimethyl-, hydrochloride. The molecular formula is C20H21N ∙ HCl and the molecular weight is 311.85 g/mol, and it has a pK of 6.8. It is freely soluble in water and pH independent. Cyclobenzaprine hydrochloride has the following chemical structure:
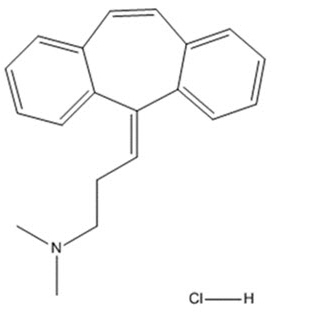
Each sublingual tablet contains 2.8 mg of cyclobenzaprine hydrochloride (equivalent to 2.47 mg cyclobenzaprine) and the following inactive ingredients: colloidal silicon dioxide, corn starch, crospovidone (Type A), D&C Yellow No.10, dibasic potassium phosphate, mannitol, and sodium stearyl fumarate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of cyclobenzaprine for the treatment of fibromyalgia in adults is unknown. In in vitro pharmacology studies, cyclobenzaprine demonstrated functional antagonism of 5-HT2A, α1-adrenergic, H1-histaminergic, and M1-muscarinic acetylcholine receptors. In addition, pharmacological studies in animals demonstrated a similarity between the effects of cyclobenzaprine and the structurally related TCAs, including reserpine antagonism, norepinephrine potentiation, potent peripheral and central anticholinergic effects, and sedation.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of cyclobenzaprine have not been fully characterized.
12.3 Pharmacokinetics
Following single-dose sublingual administration of 2.8 mg and 5.6 mg of TONMYA in healthy adult subjects (n=16), Cmax, AUC0-tau, and AUC0-inf of cyclobenzaprine increased in an approximately dose-proportional manner.
In a multiple-dose study of 5.6 mg of TONMYA administered sublingually once daily for 20 days in healthy subjects (n=26), approximately 2.5-fold accumulation of plasma cyclobenzaprine exposure was observed at steady-state.
Absorption
The median time to peak plasma cyclobenzaprine concentration (Tmax) was approximately 4.3 hours for the 2.8 mg and 5.6 mg once daily TONMYA dosages.
Food Effect: A food effect study was conducted in healthy adult subjects (n=16) with a single sublingual 5.6 mg TONMYA dose. When TONMYA was administered with food, there was approximately 10% decrease in peak plasma concentration (Cmax) compared to the fasted state with no effect on exposure (AUC0-tau and AUC0-inf). These changes are not clinically significant.
Elimination
TONMYA has a single dose elimination half-life of approximately 36 hours (range 28-59 hours; n=16).
Metabolism: Cyclobenzaprine is extensively metabolized. Cytochromes P-450 3A4, 1A2, and, to a lesser extent, 2D6 mediate N-demethylation, one of the oxidative pathways for cyclobenzaprine metabolism.
Excretion: Cyclobenzaprine is excreted primarily as glucuronides via the kidney.
Specific Populations
Geriatric Patients: After cyclobenzaprine extended-release capsules dosing, the plasma cyclobenzaprine AUC was increased by 40% and the half-life of cyclobenzaprine was prolonged in geriatric subjects greater than 65 years of age compared to younger adult subjects 18 to 45 years of age (50 hours vs. 32 hours, respectively) [see Use in Specific Populations (8.5)]. There were no notable differences in Cmax or Tmax in this study.
Hepatic Impairment: In a pharmacokinetic study of immediate-release cyclobenzaprine tablets in 15 patients with mild hepatic impairment (HI) (Child-Pugh A) and 1 patient with moderate HI (Child-Pugh B), both AUC and Cmax were approximately double the values seen in subjects with normal hepatic function. The pharmacokinetics of cyclobenzaprine in patients with moderate HI (1 patient only) and severe HI is not known [see Use in Specific Populations (8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies were conducted in CD-1 mice and Sprague-Dawley rats with oral cyclobenzaprine to evaluate its carcinogenic potential. In an 81-week carcinogenicity study, metastatic hemangiosarcoma was seen in 3 of 21 male mice at 10 mg/kg/day (approximately 9 times the maximum recommended human dose (MRHD) of 5.6 mg of TONMYA on a mg/m2 basis). In a 105-week carcinogenicity study, malignant astrocytoma was seen in 3 of 50 male rats at 10 mg/kg/day (approximately 0.3 times the MRHD on an estimated AUC basis). There were no tumor findings in female mice or rats.
Mutagenesis
Cyclobenzaprine was not mutagenic or clastogenic in the following assays: in vitro Ames bacterial mutation assays, an in vitro Chinese hamster ovary (CHO) cell chromosomal aberration test, an in vitro CHO cell micronucleus test, and in vivo mouse bone marrow micronucleus assays.
Impairment of Fertility
Cyclobenzaprine HCl, when administered 70 and 14 days prior to mating to male and female rats, respectively, had no effects on fertility or reproductive performance at oral doses up to 20 mg/kg/day (approximately 1.1 and 3.8 times in males and females, respectively, the MRHD on an estimated AUC basis).
13.2 Animal Toxicology and/or Pharmacology
In a 67-week study with rats that received cyclobenzaprine at oral doses of 10, 20, or 40 mg/kg/day (approximately 0.3 to 4.3 times and 1.2 to 12.4 times in males and females, respectively, the maximum recommended human dose (MRHD) of 5.6 mg of TONMYA on an estimated AUC basis), there were findings in the liver consisting of midzonal vacuolation with lipidosis for males and midzonal and centrilobular hepatocytic enlargement for females. In addition, there were findings of centrilobular coagulative necrosis. In the higher dose groups, these microscopic changes were seen after 26 weeks and even earlier in rats that died prior to 26 weeks; at lower doses, these changes were not seen until after 26 weeks.
In a 26-week study with Cynomolgus monkeys that received cyclobenzaprine at oral of doses of 2.5, 5, 10, or 20 mg/kg/day, one monkey at 20 mg/kg/day (69 times the MRHD on mg/m2 basis) was euthanized in week 17. Morbidity for this animal was attributed to findings of chronic pancreatitis, cholecystitis, cholangitis, and focal liver necrosis.
-
14 CLINICAL STUDIES
The efficacy of TONMYA for the treatment of fibromyalgia was assessed in three randomized, two-arm, parallel-group, double-blind, placebo-controlled multicenter trials (Trial 1 [NCT04172831], Trial 2 [NCT04508621], and Trial 3 [NCT05273749]). The trials enrolled 1,474 patients aged 18 to 65 (735 TONMYA-treated patients and 739 placebo-treated patients) who met the 2016 American College of Rheumatology (ACR) criteria for diagnosis of fibromyalgia (including generalized pain, defined as pain in at least 4 of 5 regions; symptoms that had been present at a similar level for at least 3 months; and widespread pain index (WPI) ≥7 and symptom severity scale (SSS) score ≥5, OR WPI between 4 to 6 and SSS scale score ≥9). In the trials, 95% of patients were female, 83% of patients were not Hispanic or Latino, and the percentage of White, Black, and Asian patients were 86%, 9%, and 1%, respectively. The mean age was 49 years (range 18 to 65 years), and the mean duration of fibromyalgia was 9 years (range 0 to 49 years).
Patients were randomized to receive bedtime sublingual treatment of either:
- 2.8 mg of TONMYA nightly for the first 2 weeks, and then 5.6 mg of TONMYA beginning on the evening of Day 15 through Week 14.
- Placebo administered through Week 14.
The primary endpoint in all three trials was the change from baseline to Week 14 in the weekly average of daily 24-hour recall pain intensity scores.
As measured by the 11-point (0-10) numeric rating scale (NRS), the minimum mean baseline pain score required for enrollment was 4. Within each trial, at baseline, the TONMYA and placebo groups had similar mean weekly averages of daily diary pain scores.
- In Trials 1 and 3, TONMYA demonstrated a statistically significant reduction in pain intensity scores as compared to placebo (Table 2).
- In Trial 2, there was no statistically significant treatment group difference (TONMYA minus placebo). Results of this trial may not have been generalizable due to the presence of factors outside the conduct of the study.
The least squares (LS) mean change from baseline in the weekly average of daily 24-hour recall pain intensity scores at Week 14 in adult patients with fibromyalgia for the TONMYA and placebo groups in Trial 1 and Trial 3 are presented in Table 2. The LS mean change from baseline in the weekly average of daily 24-hour recall pain intensity scores over the weeks of the study in adult patients with fibromyalgia in Trials 1 and 3 are presented in Figure 1.
Table 2: Primary Efficacy Endpoint: Mean Change from Baseline in Weekly Average of Daily 24-Hour Recall Pain Intensity Scores at Week 14 in Adult Patients with Fibromyalgia (Trials 1 and 3) Placebo TONMYA Visit/
StatisticsValue Change from baseline Value Change from baseline CI = confidence interval; LS = least squares; SD = standard deviation; SE = standard error - * LS means, differences and CIs were based on a mixed model for repeated measures with fixed, categorical effects of treatment, center, study week, and treatment-by-study week interaction, as well as the fixed covariates of baseline value and baseline value-by-study week interactions. An unstructured covariance matrix was used.
- † The difference in the LS mean (i.e., -0.7) was due to a rounding effect: the change from baseline was -1.82 in the TONMYA group and -1.16 in the placebo group, and the difference in LS mean between the groups was -0.66.
Trial 1
BaselineN 255 248 Mean (SD) 6.0 (1.08) 6.1 (1.06) (Minimum, Maximum) (4, 9) (4, 9) Week 14 LS mean (SE)* 4.6 (0.12) -1.5 (0.12) 4.2 (0.12) -1.9 (0.12) 95% CI* (4.3, 4.8) (-1.7, -1.3) (3.9, 4.4) (-2.1, -1.7) Difference in LS mean (SE) -0.4 (0.16) 95% CI for difference in LS mean (-0.7, -0.1) p-value for difference 0.010 Trial 3
BaselineN 225 231 Mean (SD) 5.9 (1.08) 5.9 (1.05) (Minimum, Maximum) (4, 9) (4, 9) Week 14 LS mean (SE)* 4.7 (0.12) -1.2 (0.12) 4.1 (0.12) -1.8 (0.12) 95% CI* (4.5, 5.0) (-1.4, -0.9) (3.8, 4.3) (-2.0, -1.6) Difference in LS mean (SE) -0.7 (0.16)† 95% CI for difference in LS mean (-1.0, -0.3) p-value for difference <0.001 Figure 1 Mean Change from Baseline in Weekly Average of Daily 24-Hour Recall Pain Intensity Scores Over Time in Adult Patients with Fibromyalgia (Trials 1 and 3)
Trial 1
Error bars represent +/- the standard error (SE). 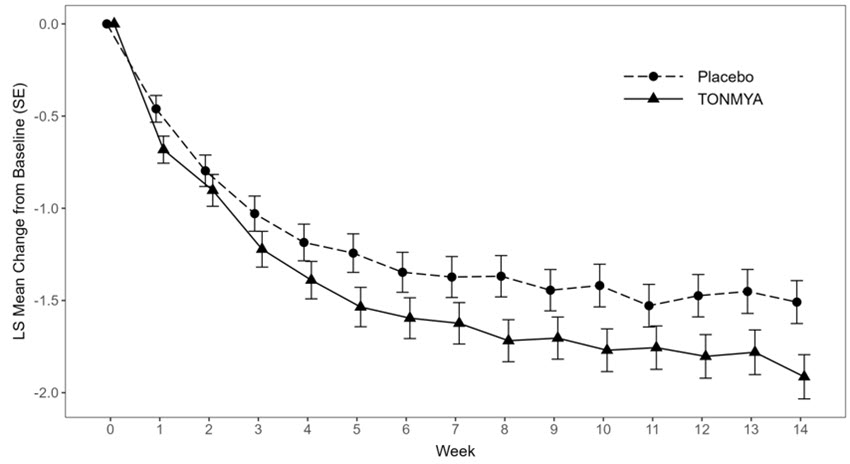
Trial 3
Error bars represent +/- the standard error (SE). 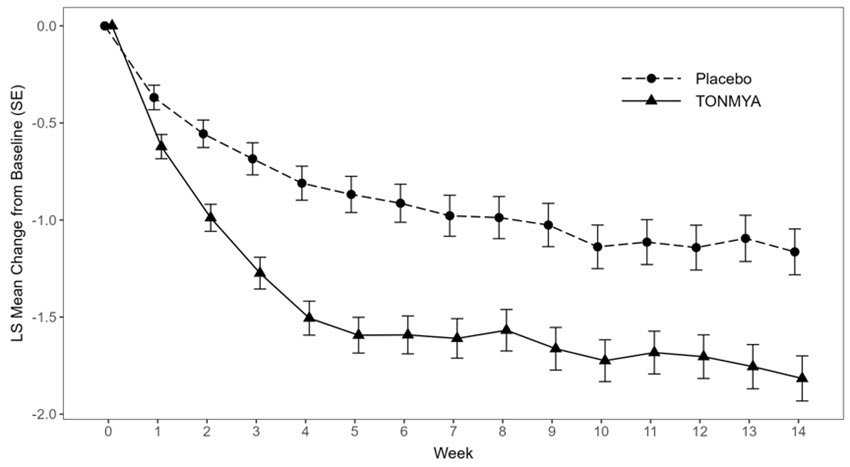
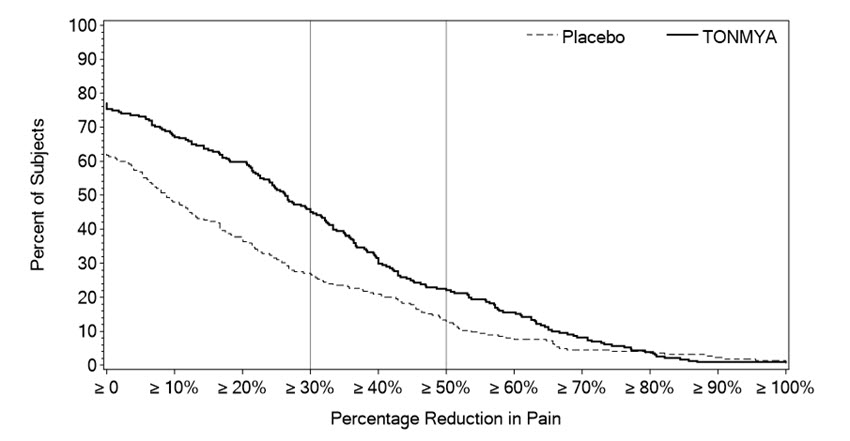
Figure 2 shows the percentage of patients in Trials 1 and 3 who achieved various degrees of improvement in the change from baseline to Week 14 in the weekly averages of daily diary pain scores. The figures are cumulative so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the trial were assigned 0% improvement. For example, in Trial 1 and Trial 3, the percentage of TONMYA-treated patients who achieved at least a 30% improvement from baseline in their weekly average of daily 24-hour recall pain intensity score at Week 14 was 47% and 46%, respectively.
Figure 2 Patients Who Achieved Various Levels of Improvement in Pain Intensity at Week 14 – Trial 1 and Trial 3
Trial 1
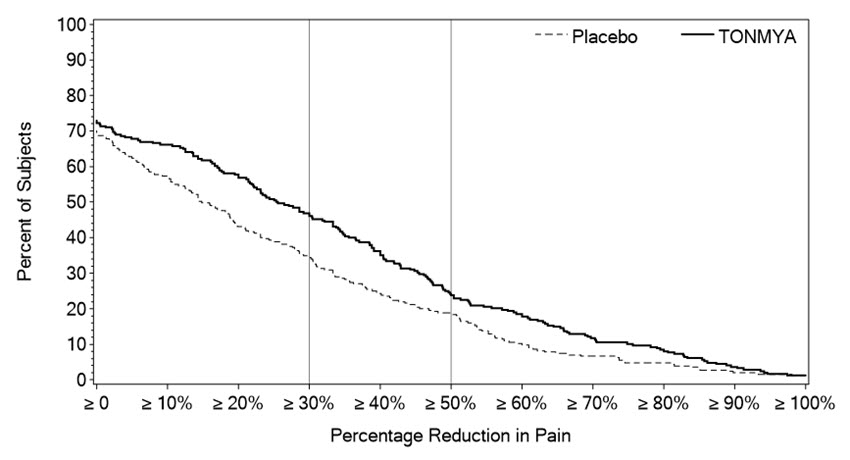
Trial 3
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TONMYA (cyclobenzaprine hydrochloride sublingual tablets) are supplied as 2.8 mg sublingual tablets and are round, yellow debossed "T" on one side and packaged in 30 cc high density polyethylene bottles with a 28 mm polypropylene child-resistant cap with induction seal. Each bottle contains polyester coil and a desiccant canister.
- 14 count bottle NDC [70792-102-14]
- 30 count bottle NDC [70792-102-30]
- 60 count bottle NDC [70792-102-60]
- 90 count bottle NDC [70792-102-90]
16.2 Storage and Handling
TONMYA (cyclobenzaprine hydrochloride sublingual tablets) are labeled for storage at USP controlled room temperature (20°C to 25°C [68°F to 77°F]) with excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Store and dispense in original container and protect from moisture. Remove the polyester coil on first use and discard. Keep the desiccant canister in the bottle for the entire period of use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Advise patients to not swallow whole, cut, crush, or chew TONMYA.
- Advise patients to moisten mouth with sips of water before administration of TONMYA to reduce the risk of oral numbness.
- Advise patients that if a dose is missed, take the next dose at the regularly scheduled time the following evening. Patients should not take two doses to make up for a missed dose.
- Advise patients to stop taking TONMYA and to notify their health care provider right away if they experience symptoms of an allergic reaction, such as difficulty breathing, hives, swelling of face or tongue, or itching.
- Advise patients that TONMYA should not be taken with MAO inhibitors or within 14 days after discontinuation of a MAO inhibitor.
- Caution patients about the risk of serotonin syndrome with concomitant use of TONMYA and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. Advise patients of the signs and symptoms of serotonin syndrome [see Warnings and Precautions (5.1)] and instruct patients to seek medical care immediately if they experience these symptoms.
- Advise patients to stop taking TONMYA and to notify their health care provider right away if they experience symptoms of arrhythmias or tachycardia.
- Advise patients that TONMYA may enhance the impairment effects of alcohol. These effects may also be seen if TONMYA is taken with other CNS depressants.
- Advise patients to report severe oral mucosal adverse reactions to their health care provider.
- Caution patients about operating a motor vehicle or hazardous machinery until it is reasonably certain that TONMYA therapy will not adversely affect their ability to engage in such activities.
- Advise patients to take TONMYA at approximately the same time each night.
- Inform female patients of reproductive potential that TONMYA may cause fetal harm and to inform their healthcare providers of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
- Advise female patients of reproductive potential to use effective contraception during treatment with TONMYA and for two weeks after the final dose [see Use in Specific Populations (8.3)].
- Advise patients who are exposed to TONMYA during pregnancy to contact Tonix Medicines, Inc., at 1-888-869-7633 (1-888-TNXPMED).
-
SPL UNCLASSIFIED SECTION
Distributed by:
Tonix Medicines, Inc.
26 Main Street – Suite 101, Chatham, NJ 07928Manufactured for:
Tonix Pharmaceuticals, Inc.
26 Main Street – Suite 101, Chatham, NJ 07928Manufactured by:
Almac Pharma Services Limited
Craigavon, Northern Ireland BT63 5QD
United Kingdom
and
Almac Pharma Services LLC
2661 Audubon Rd
Audubon, PA 19403-2413, USA
and
Patheon Inc.
111 Consumers Drive
Whitby, Ontario L1N 5Z5, Canada -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
TONMYA™ (ton mye ah)
(cyclobenzaprine hydrochloride sublingual tablets)This Patient Information has been approved by the U.S. Food and Drug Administration. Issued MM/2025 What is TONMYA?
TONMYA is a prescription medicine used in adults for the treatment of fibromyalgia.
It is not known if TONMYA is safe and effective in children.Do not use TONMYA if you: - are allergic to cyclobenzaprine or any of the ingredients in TONMYA. See the end of the Patient Information leaflet for a complete list of ingredients in TONMYA.
Stop taking TONMYA and call your healthcare provider or get medical help right away if you have symptoms of an allergic reaction such as:
- difficulty breathing
- hives
- swelling of your face or tongue
- itching
- are taking, or have stopped taking within the last 14 days, a medicine called monoamine oxidase (MAO) inhibitor. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
- have had a recent heart attack, have heart rhythm problems (arrhythmias) or heart failure
- have an overactive thyroid (hyperthyroidism)
Before taking TONMYA, tell your healthcare provider about all of your medical conditions, including if you: - have a history of seizures
- have trouble emptying your bladder (urinary retention)
- have a history of eye problems including glaucoma
- have liver problems
- are pregnant or plan to become pregnant. TONMYA may harm your unborn baby. You should not take TONMYA if you are planning to become pregnant or during the first trimester (first 12 weeks) of pregnancy.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with TONMYA.
- Use effective birth control (contraception) during treatment with TONMYA and for 2 weeks after the final dose.
- Tell your healthcare provider right away if you are planning to become pregnant, you become pregnant, or think you are pregnant during treatment with TONMYA.
- If you are exposed to TONMYA during pregnancy, contact Tonix Medicine, Inc., at 1-888-869-7633 (1-888-TNXPMED).
- are breastfeeding or plan to breastfeed. TONMYA may pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with TONMYA.
Especially tell your healthcare provider if you take:- a medicine to treat depression, mood, anxiety, psychotic, or thought disorders
- a pain medicine called tramadol or meperidine
- bupropion
- verapamil
- anticholinergic medicines
- barbiturates or other medicines that depress your central nervous system (CNS depressants)
- a blood pressure medicine called guanethidine
Know the medicines you take. Keep a list of your medicines with you and show it to your healthcare provider or pharmacist when you get a new medicine.How should I take TONMYA? - Take TONMYA exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much TONMYA to take.
- Your healthcare provider may change your TONMYA dose if needed.
- Take TONMYA 1 time a day at bedtime, around the same time each evening.
- Take TONMYA after brushing your teeth and other oral care is completed.
- Your mouth and area under your tongue (sublingual) should be moist when you take TONMYA. Drink a few sips of water before you take TONMYA.
- Place the prescribed number of TONMYA sublingual tablet(s) under your tongue until it dissolves.
- Do not swallow whole, cut, crush, or chew TONMYA sublingual tablets.
- Avoid eating or drinking for at least 15 minutes after the TONMYA sublingual tablet is completely dissolved. Avoid any hot, cold, or acidic beverages until the morning.
- Avoid talking for at least 5 minutes after taking TONMYA.
- If you miss a dose of TONMYA, take your next dose the following evening. Do not take a missed dose during the day. Do not take 2 doses to make up for a missed dose.
- If you take too much TONMYA, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What should I avoid while using TONMYA? - You should not drink alcohol until you know how TONMYA affects you. Taking TONMYA with alcohol or other medicines that depress your central nervous system can slow your thinking and physical response times, and cause symptoms such as sleepiness.
- Do not drive, operate machinery, or do other dangerous activities until you know how TONMYA affects you.
What are possible side effects of TONMYA?
TONMYA may cause serious side effects, including:-
Serotonin syndrome. Serotonin syndrome is a serious condition that may be life-threatening and has happened when TONMYA is used with certain other medicines. Go to the nearest hospital emergency room right away if you develop any of following signs or symptoms of serotonin syndrome:
- confusion, agitation, seeing or hearing things that are not real (hallucinations), coma, or other changes in mental status
- high body temperature (hyperthermia)
- shaking (tremor), coordination problems, or muscle twitching (overactive reflexes)
- fast heartbeat, high or low blood pressure
- sweating or fever
- nausea, vomiting, or diarrhea
- muscle stiffness or tightness
- TONMYA may cause serious side effects that may lead to heart attack or stroke. Stop taking TONMYA and go to the nearest hospital emergency room right away if you get:
- irregular or abnormal heartbeats (arrhythmias)
- fast heartbeat (tachycardia)
- dizziness
- lightheaded or feel faint
- chest pain or discomfort
- shortness of breath
-
Side effects in your mouth. TONMYA can cause side effects to the lining inside your mouth, including numbness, tingling, discomfort, pain, irritation, swelling (inflammation), and sores (lesions). Side effects inside your mouth are common with TONMYA and can sometimes be severe. These side effects can happen within minutes of taking TONMYA, and most go away within 60 minutes. Drink a few sips of water before taking TONMYA to decrease your chance of getting numbness or reduced feeling in your mouth. Tell your healthcare provider right away if you have:
- trouble eating, drinking, breathing, or swallowing
- side effects that cover a large area in your mouth
- pain in your mouth that makes it difficult to do your normal activities
- reduced feeling in your mouth
- mouth discomfort
- abnormal TONMYA taste
- feeling sleepy (somnolence)
- numbness or tingling in your mouth
- mouth pain
- tiredness
- dry mouth
- sores in your mouth (canker sores)
These are not all the possible side effects of TONMYA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store TONMYA? - Store TONMYA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TONMYA in the original bottle. Protect from moisture.
- The TONMYA bottle contains a desiccant canister to help keep your medicine dry (protect it from moisture). Keep the desiccant canister in the bottle.
- The TONMYA bottle contains a polyester coil to help protect the sublingual tablets during shipping. Remove the polyester coil from the bottle and throw it away after opening the bottle for the first time.
- TONMYA comes in a child-resistant bottle.
General information about the safe and effective use of TONMYA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TONMYA for a condition for which it was not prescribed. Do not give TONMYA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TONMYA that is written for health professionals.What are the ingredients in TONMYA?
Active ingredient: cyclobenzaprine hydrochloride
Inactive ingredients: colloidal silicon dioxide, corn starch, crospovidone (Type A), D&C Yellow No.10, dibasic potassium phosphate, mannitol, and sodium stearyl fumarate.
Distributed by: Tonix Medicines, Inc., 26 Main Street – Suite 101, Chatham, NJ 07928
Manufactured for: Tonix Pharmaceuticals, Inc., 26 Main Street – Suite 101, Chatham, NJ 07928
Manufactured by: Almac Pharma Services Limited, Craigavon, Northern Ireland BT63 5QD, United Kingdom and Almac Pharma Services LLC, 2661 Audubon Rd, Audubon, PA 19403-2413, USA and Patheon Inc., 111 Consumers Drive, Whitby, Ontario L1N 5Z5, Canada
For optional information, visit www.TONMYA.com or call 1-888-869-7633 (1-888-TNXPMED). - are allergic to cyclobenzaprine or any of the ingredients in TONMYA. See the end of the Patient Information leaflet for a complete list of ingredients in TONMYA.
-
PRINCIPAL DISPLAY PANEL - 2.8 mg Tablet Bottle Label
NDC: 70792-102-30
30 TabletsRx ONLY
Tonmya™
(cyclobenzaprine HCl
sublingual tablets)Distributed by:
Tonix Medicines, Inc.
Chatham, NJ 079282.8 mg
TONIX
MEDICINES
-
INGREDIENTS AND APPEARANCE
TONMYA
cyclobenzaprine hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70792-102 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cyclobenzaprine Hydrochloride (UNII: 0VE05JYS2P) (Cyclobenzaprine - UNII:69O5WQQ5TI) Cyclobenzaprine Hydrochloride 2.8 mg Inactive Ingredients Ingredient Name Strength Mannitol (UNII: 3OWL53L36A) Starch, Corn (UNII: O8232NY3SJ) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color YELLOW Score no score Shape ROUND Size 3mm Flavor Imprint Code T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70792-102-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2025 2 NDC: 70792-102-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2025 3 NDC: 70792-102-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2025 4 NDC: 70792-102-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219428 08/15/2025 Labeler - Tonix Medicines, Inc. (080269086) Registrant - Tonix Pharmaceuticals, Inc. (829472302) Establishment Name Address ID/FEI Business Operations Almac Pharma Services Limited 233170864 MANUFACTURE(70792-102) Establishment Name Address ID/FEI Business Operations Patheon Inc. 205475333 MANUFACTURE(70792-102) , LABEL(70792-102)
Trademark Results [TONMYA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TONMYA 97185424 not registered Live/Pending |
TONIX PHARMACEUTICALS, INC. 2021-12-22 |
 TONMYA 86516050 4868328 Live/Registered |
TONIX PHARMACEUTICALS, INC. 2015-01-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.