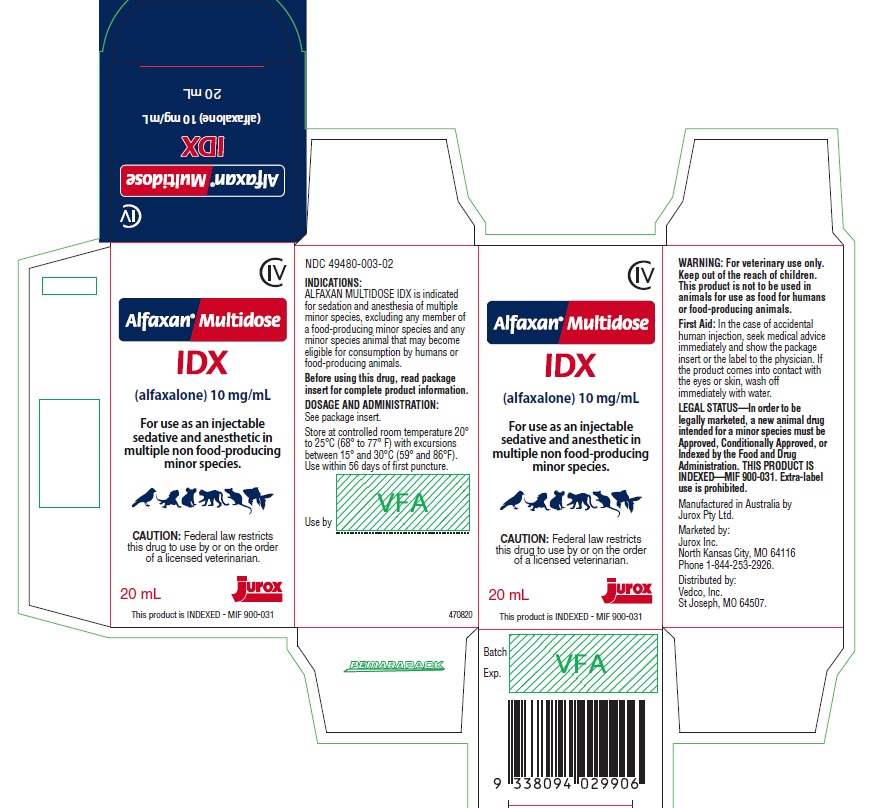
ALFAXAN MULTIDOSE IDX- alfaxalone solution
ALFAXAN by
Drug Labeling and Warnings
ALFAXAN by is a Animal medication manufactured, distributed, or labeled by Jurox Pty. Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
GENERAL PRECAUTIONS
FOR ANIMAL USE ONLY
Alfaxan® Multidose IDX CIV
(alfaxalone) 10 mg/mL Injectable Solution
For use as an injectable sedative and anesthetic in multiple nonfood-producing minor species.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
LEGAL STATUS: In order to be legally marketed, a new animal drug intended for a minor species must be Approved, Conditionally Approved, or Indexed by the Food and Drug Administration. THIS PRODUCT IS INDEXED—MIF 900-031. Extra-label use is prohibited.
This product is not to be used in animals for use as food for humans or food-producing animals. -
DESCRIPTION
DESCRIPTION
ALFAXAN MULTIDOSE IDX contains alfaxalone, a neuroactive steroid molecule with properties of a general anesthetic. Alfaxalone is chemically described as 3‑ α‑ hydroxy‑ 5‑ α‑ pregnane‑ 11, 20‑dione, and has a molecular weight of 332.5. The primary mechanism for the anesthetic action of alfaxalone is modulation of neuronal cell membrane chloride ion transport, induced by binding of alfaxalone to GABAA (gamma‑aminobutyric acid) cell surface receptors. This product contains the following preservatives: chlorocresol (0.1% w/v), benzethonium chloride (0.02% w/v) and ethanol (15% w/v).
-
INDICATIONS & USAGE
INDICATIONS
ALFAXAN MULTIDOSE IDX is indicated as a sedative and anesthetic in multiple minor species*. More specifically, ALFAXAN MULTIDOSE IDX is indicated for the following:
- For sedation and anesthesia in captive reptiles, excluding any food‑producing species**
- For sedation and anesthesia in captive amphibians, excluding any food‑producing species**
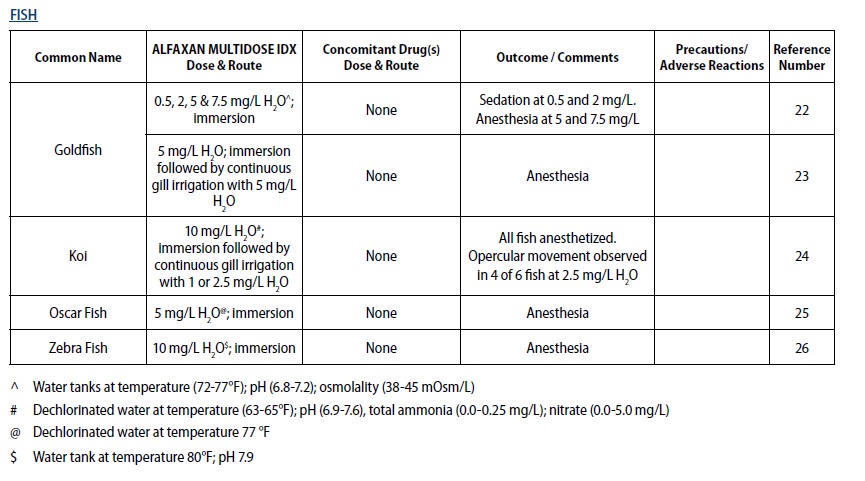
- For sedation and anesthesia in ornamental fish, including species used in research such as the zebra fish
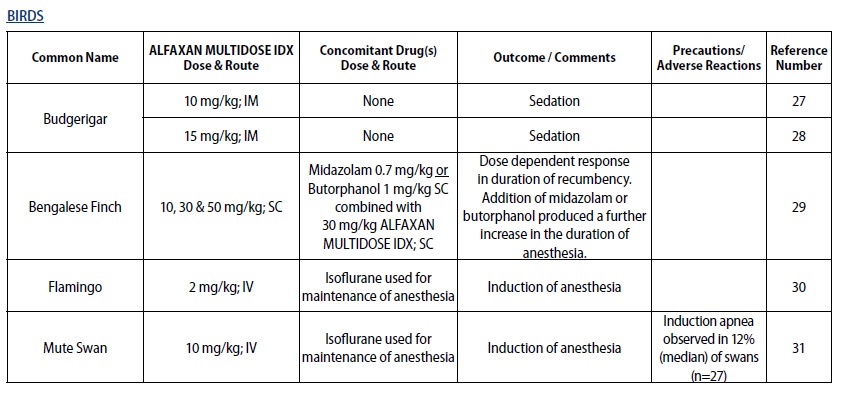
- For sedation and anesthesia in captive species and pet birds in the orders Psittaciformes, Passeriformes, and Columbiformes, excluding any food‑producing species**
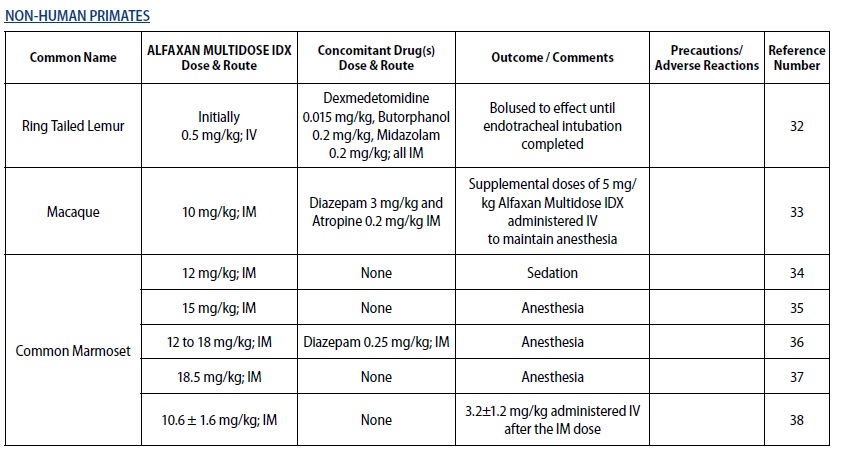
- For sedation and anesthesia in non‑human primates
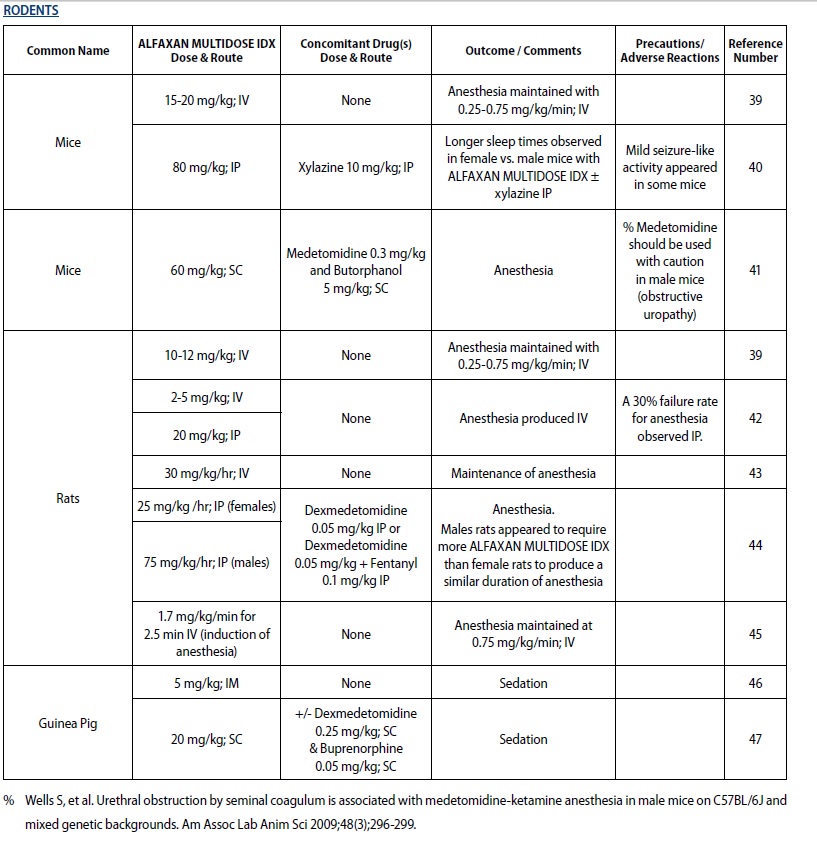
- For sedation and anesthesia in captive rodents
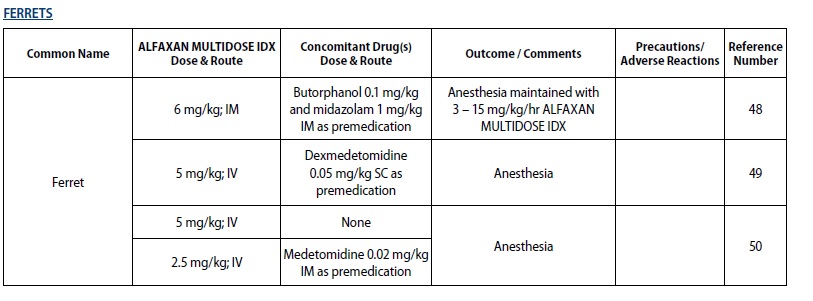
- For sedation and anesthesia in captive mustelids
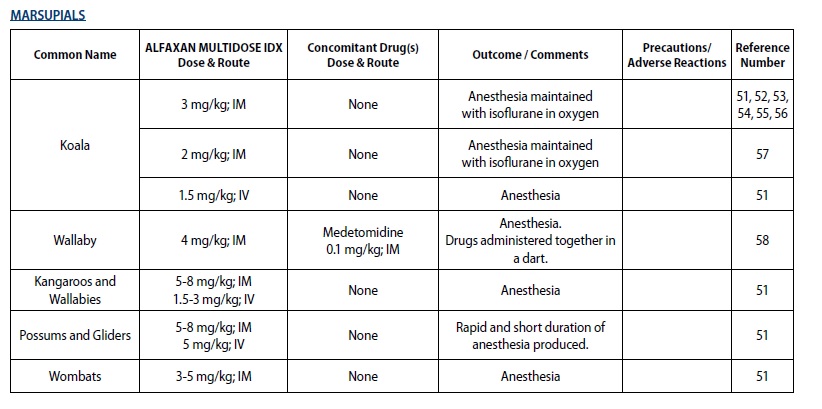
- For sedation and anesthesia in captive marsupials
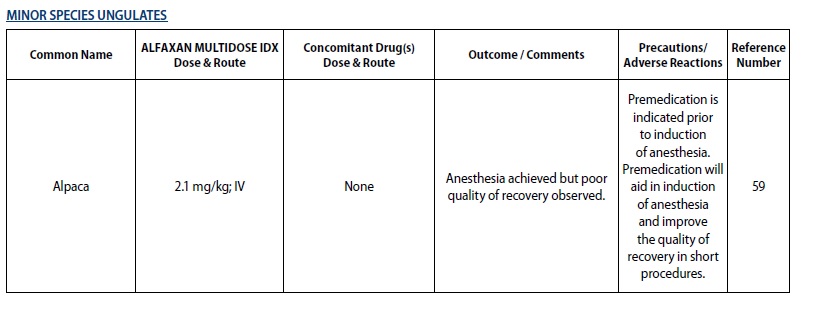
- For induction of anesthesia and immobilization in captive minor species ungulates, excluding any food‑producing species**
Use only when there is reasonable certainty that the treated animal will not be consumed by humans or food‑producing animals.
* The term “minor species” means animals other than humans that are not major species. “Major species” means cattle, horses, swine, chickens, turkeys, dogs and cats.
** As used on this label, a “food‑producing minor species” is considered to be a minor species of which some members are bred, cultured, farmed, ranched, hunted, caught, trapped or otherwise harvested for the purpose of having the animals or edible products of the animals commercially distributed for consumption by humans or food‑producing animals in the United States.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
When administering ALFAXAN MULTIDOSE IDX by intravenous injection administer slowly to effect, titrating administration against the response of the patient. Rapid administration of ALFAXAN MULTIDOSE IDX may be associated with an increased incidence of cardiorespiratory depression or apnea. The use of preanesthetics may reduce the ALFAXAN MULTIDOSE IDX induction dose. The choice and the amount of phenothiazine, alpha₂‑ adrenoreceptor agonist, benzodiazepine or opioid will influence the response of the patient to an induction dose of ALFAXAN MULTIDOSE IDX.
When using ALFAXAN MULTIDOSE IDX, patients should be continuously monitored, and facilities for the maintenance of a patent airway, artificial ventilation, and oxygen supplementation must be immediately available.
ALFAXAN MULTIDOSE IDX contains preservatives. Use within 56 days of first puncture. Any unused ALFAXAN MULTIDOSE IDX remaining after 56 days should be discarded.
The following tables outline the dosage and administration of ALFAXAN MULTIDOSE IDX for the indicated species by major group. The doses are representative of doses published in the literature. Veterinarians are advised to consult the published literature before use of the product (see List of References at end of product insert).
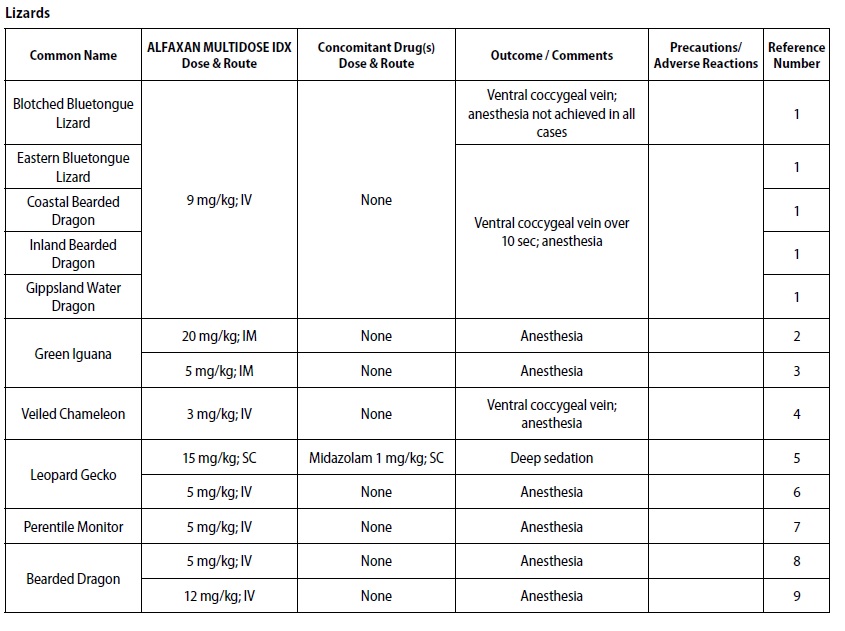
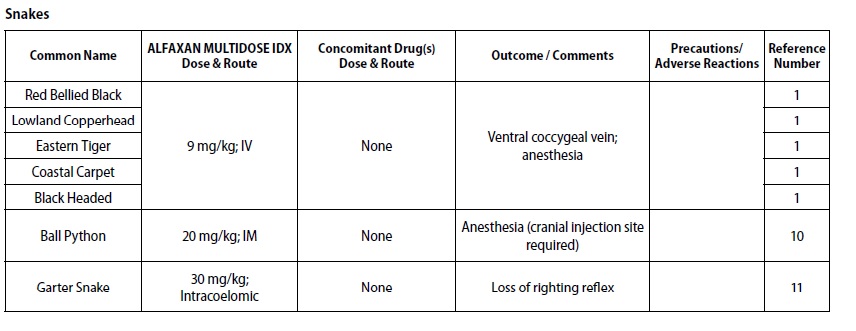
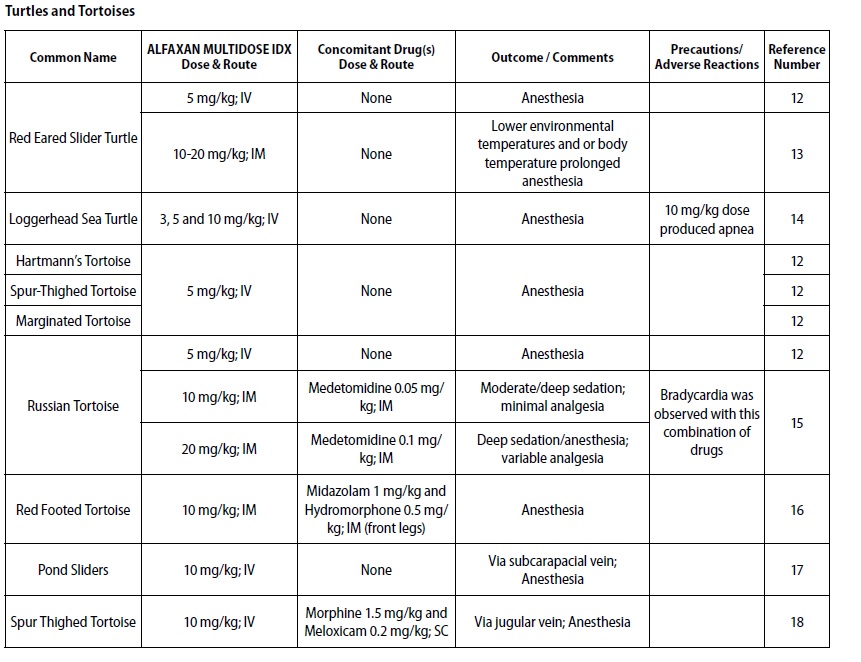
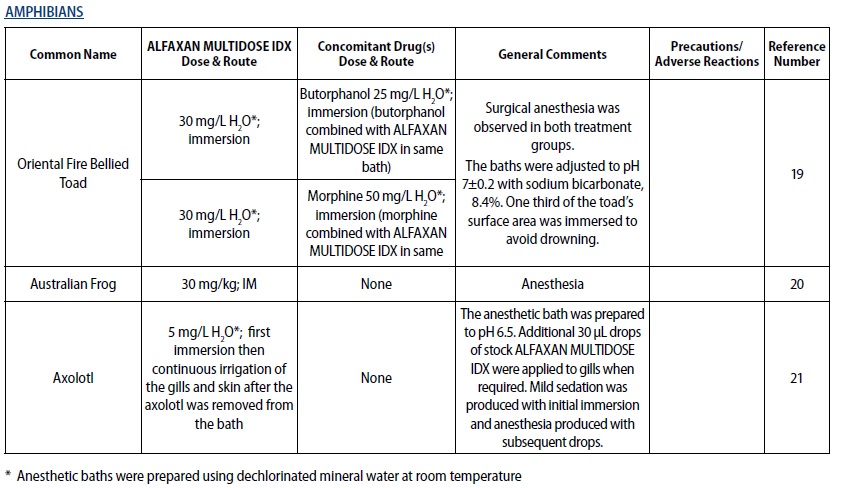
- REPTILES
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
-
DRUG INTERACTIONS
DRUG INTERACTIONS
No specific preanesthetic is either indicated or contraindicated with ALFAXAN MULTIDOSE IDX. The necessity for and choice of preanesthetic is left to the discretion of the veterinarian. Preanesthetic doses may be lower than the label directions for their use as a single medication. ALFAXAN MULTIDOSE IDX is compatible with benzodiazepines, opioids, alpha2‑agonists, and phenothiazines as commonly used in surgical practice.
-
CONTRAINDICATIONS
CONTRAINDICATIONS
ALFAXAN MULTIDOSE IDX is contraindicated in animals with a known sensitivity to ALFAXAN MULTIDOSE IDX or its components, or when general anesthesia and/or sedation are contraindicated. Do not use in any minor species animal that may become eligible for consumption by humans or food‑producing animals.
-
WARNINGS
WARNINGS
Animal Safety: Rapid bolus administration or anesthetic overdose may cause cardiorespiratory depression, including hypotension, apnea, hypoxia, or death. Arrhythmias may occur secondary to apnea and hypoxia. In cases of anesthetic overdose, stop ALFAXAN MULTIDOSE IDX administration and administer treatment as indicated by the patient’s clinical signs. Cardiovascular depression should be treated with plasma expanders, pressor agents, anti‑arrhythmic agents or other techniques as appropriate for the treatments of the clinical signs
Human safety: Not for human use. Keep out of the reach of children.
ALFAXAN MULTIDOSE IDX should be managed to prevent the risk of diversion, through such measures as restriction of access and the use of drug accountability procedures appropriate to the clinical setting.
Exercise caution to avoid accidental self‑injection. Overdose is likely to cause cardiorespiratory depression (such as hypotension, bradycardia and/ or apnea). Remove the individual from the source of exposure and seek medical attention. Respiratory depression should be treated by artificial ventilation and oxygen. Avoid contact of this product with skin, eyes, and clothes. In case of contact, eyes and skin should be liberally flushed with water for 15 minutes. Consult a physician if irritation persists. In the case of accidental human ingestion, seek medical advice immediately and show the package insert or the label to the physician.
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report adverse reactions in users or to obtain a copy of the SDS for this product call 1‑844‑253‑2926.
Note to physician: This product contains an injectable anesthetic.
-
DRUG ABUSE AND DEPENDENCE
DRUG ABUSE AND DEPENDENCE
Controlled substance: ALFAXAN MULTIDOSE IDX contains alfaxalone, a neurosteroid anesthetic and a class IV controlled substance.
Abuse: Alfaxalone is a central nervous system depressant that acts on GABA receptor associated chloride channels, similar to the mechanism of action of Schedule IV sedatives such as benzodiazepines (diazepam and midazolam), barbiturates (phenobarbital and methohexital) and fospropofol. In a drug discrimination behavioral test in rats, the effects of alfaxalone were recognized as similar to those of midazolam. These biochemical and behavioral data suggest that alfaxalone has an abuse potential similar to other Schedule IV sedatives.
Physical dependence: There are no data that assess the ability of alfaxalone to inducephysical dependence. However, alfaxalone has a mechanism of action similar to the benzodiazepines and can block the behavioral responses associated with precipitated benzodiazepine withdrawal. Therefore, it is likely that alfaxalone can also produce physical dependence and withdrawal signs similar to that produced by the benzodiazepines
Psychological dependence: The ability of alfaxalone to produce psychological dependence is unknown because there are no data on the rewarding properties of the drug from animal self‑administration studies or from human abuse potential studies.
-
PRECAUTIONS
PRECAUTIONS
Analgesia during anesthesia: ALFAXAN MULTIDOSE IDX is not an analgesic and appropriate analgesia should be provided to the patient for painful procedures.
Rapid arousal: Careful monitoring of the patient is necessary due to possibility of rapid arousal.
Apnea: Apnea may occur following IV administration of an induction dose, maintenance dose or a dose administered during transition to inhalant maintenance anesthesia of ALFAXAN MULTIDOSE IDX, especially with higher doses and rapid administration. Endotracheal intubation, oxygen supplementation and intermittent positive pressure ventilation (IPPV) should be administered to treat apnea and associated hypoxemia in the appropriate species.
Blood Pressure: ALFAXAN MULTIDOSE IDX can exacerbate the myocardial depressive and vasodilatory effects of inhalant anesthetics resulting in hypotension. Preanesthetics can potentiate the effect of ALFAXAN MULTIDOSE IDX resulting in more pronounced changes in blood pressure. Transient hypertension has also been observed with ALFAXAN MULTIDOSE IDX administration, possibly due to elevated sympathetic activity in the patient. It is prudent to monitor blood pressure whenever possible.
Body temperature: Steps should be taken to maintain the normal physiological temperature of the patient during anesthesia. Supplemental heat, appropriate for the species, should be provided to maintain acceptable core body temperature until full recovery.
Breeding animals: Alfaxalone crosses the placenta, and as with other general anesthetic agents, the administration of ALFAXAN MULTIDOSE IDX may be associated with neonatal depression.
Compromised or debilitated animals: Caution should be used in animals with cardiac, respiratory, renal or hepatic impairment, or in hypovolemic or debilitated animals and geriatric animals.
-
ADVERSE REACTIONS
ADVERSE REACTIONS
Specific adverse reactions described in the referenced literature are listed in the Dosage and Administration section of the product insert.
To report adverse reactions or to obtain a copy of the SDS for this product call 1‑844‑253‑2926. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1‑888‑FDA‑VETS or online at http://www.fda.gov/reportanimalae.
-
OVERDOSAGE
OVERDOSE
Rapid administration, accidental overdose, or relative overdose due to inadequate dose sparing of ALFAXAN MULTIDOSE IDX in the presence of preanesthetics may cause cardiopulmonary depression. Respiratory arrest (apnea) may be observed. In cases of respiratory depression, stop drug administration, establish a patent airway, and initiate assisted or controlled ventilation with pure oxygen. Cardiovascular depression should be treated with plasma expanders, pressor agents, antiarrhythmic agents or other techniques as appropriate for the observed abnormality.
-
STORAGE AND HANDLING
STORAGE INFORMATION: Store at controlled room temperature 20°C ‑ 25°C (68° to 77°F) with excursions between 15° and 30°C (59° and 86°F). ALFAXAN MULTIDOSE IDX contains preservatives. The product can be used for 56 days after broaching the vial. Any unused ALFAXAN MULTIDOSE IDX remaining after 56 days should be discarded.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
REFERENCES
LIST OF REFERENCES
- Scheelings TF, Baker RT, Hammersley G, Hollis K, Elton I, Holz P. A preliminary investigation into the chemical restraint of selected Australian squamate species with Alfaxalone. J Herpetol Med Surg 2011;21(2‑3): 63‑77.
- Bertelsen MF, Sauer CD. Alfaxalone anaesthesia in the green iguana (Iguana iguana). Vet Anaesth Analg 2011;38(5):461‑6.
- Knotek Z, Hrdá A, Knotková Z, Trnková S, Babák V. Alfaxalone anaesthesia in the green iguana (Iguana iguana). ACTA VET BRNO 2013;82:109–114.
- Rowland MN. Fibrosing myopathy of the temporal muscles causing lockjaw in a veiled chameleon (Chamaeleo calyptratus). Vet Rec 2011;169:527b.
- Doss GA, Fink DM, Sladky KK, Mans C. Comparison of subcutaneous dexmedetomidine‑ midazolam versus alfaxalone‑midazolam sedation in leopard geckos (Eublepharis macularius). Vet Anaesth Analg 2017;44(5):1175‑1183.
- Morici M, DiGiuseppe M, Spadola F, Oliveri M, Knotkova Z, Knotek Z. Intravenous alfaxalone anaesthesia in leopard geckos (Eublepharis macularius). J Exot Pet Med 2018;27(3)11‑14.
- Hoffman EM, Garrett K, Doneley RJT. Pneumocoelom causing cloacal prolapse in a perentie monitor (Varanus giganteus). Australian Veterinary Practitioner 2017;47(4):117‑120.
- Knotek Z. Induction to inhalation anaesthesia in agamid lizards with alfaxalone. Veterinarni Medicina 2017;62(01):41–43.
- Perrin KL, Bertelsen MF. Intravenous alfaxalone and propofol anesthesia in the bearded dragon (Pogona vitticeps). Herpetol Med Surg 2017;27(3‑4):123‑126.
- James LE, Williams CJA, Bertelsen MF, Wang T. Anesthetic induction with alfaxalone in the ball python (Python regius): dose response and effect of injection site. Vet Anaesth Analg 2018;45(3):329‑337.
- Strahl‑Heldreth D, Clark‑Price SC, Keating SCJ, Graham LF, Chinnadurai SK, Schaeffer. Effect of intracoelomic administration of alfaxalone on the righting reflex and tactile stimulus response of common garter snakes (Thamnophis sirtalis). Am J Vet Res 2019;80(2):144‑151.
- Knotek Z. Alfaxalone as an induction agent for anaesthesia in terrapins and tortoises. Vet Rec 2014;175(13):327.
- Kischinovsky M, Duse A, Wang T, Bertelsen MF. Intramuscular administration of alfaxalone in red‑eared sliders (Trachemys scripta elegans)‑effects of dose and body temperature. Vet Anaesth Analg 2013;40(1):13‑20.
- Phillips BE, Posner LP, Lewbart GA, Christiansen EF, Harms CA. Effects of alfaxalone administered intravenously to healthy yearling loggerhead sea turtles (Caretta caretta) at three different doses. J Am Vet Med Assoc 2017;250(8):909‑917.
- Hansen LL, Bertelsen MF. Assessment of the effects of intramuscular administration of alfaxalone with and without medetomidine in Horsfield’s tortoises (Agrionemys horsfieldii). Vet Anaesth Analg 2013;40(6):e68‑75.
- Romeijer C, Beaufrère H, Laniesse D, Birch SM, MacKenzie S, Melville L, Moens N. Vomiting and gastrointestinal obstruction in a red‑footed tortoise (Chelonoidis carbonaria). J Herpetol Med Surg 2016;26(1–2):32‑35.
- Perpiñán D, Martínez‑Silvestre A, Bargalló F, Di Giuseppe M, Orós J, Costa T. Correlation between endoscopic sex determination and gonad histology in pond sliders, Trachemys scripta (Reptilia: Testudines: Emydidae). Acta Herpetologica 2016;11(1): 91‑94.
- Hedley J, Woods S, Eatwell K. The use of negative pressure wound therapy following subcarapacial abscess excision in a tortoise. J Small Anim Pract 2013;54(11):610‑613.
- Adami C, d’Ovidio D, Casoni D. Alfaxalone‑butorphanol versus alfaxalone‑morphine combination for immersion anaesthesia in oriental fire‑bellied toads (Bombina orientalis). Lab Anim 2016;50(3):204‑211.
- Sladakovic I, Johnson RS, Vogelnest L. Evaluation of intramuscular alfaxalone in three Australian frog species (Litoria caerulea, Litoria aurea, Litoria booroolongensis). J Herpetol Med Surg 2014;24(1–2):36‑42.
- McMillan MW, Leece EA. Immersion and branchial/transcutaneous irrigation anaesthesia with alfaxalone in a Mexican axolotl. Vet Anaesth Analg 2011;38(6):619‑23.
- Bauquier SH, Greenwood J, Whittem, T. Evaluation of the sedative and anaesthetic effects of five different concentrations of alfaxalone in goldfish, Carassius auratus. Aquaculture 2013;396‑399:119–123.
- O’Hagan BJ, Raidal SR. Surgical removal of retrobulbar hemangioma in a goldfish (Carassius auratus). Vet Clin North Am Exot Anim Pract 2006;9(3):729‑33.
- Minter LJ, Bailey KM, Harms CA, Lewbart GA, Posner LP. The efficacy of alfaxalone for immersion anesthesia in koi carp (Cyprinus carpio). Vet Anaesth Analg 2014;41(4):398‑405.
- Bugman AM, Langer PT, Hadzima E, Rivas AE, Mitchell MA. Evaluation of the anesthetic efficacy of alfaxalone in oscar fish (Astronotus ocellatus). Am J Vet Res 2016; 77(3):239‑244.
- Farry T, Lau C, Keates H, McEwen M, Woldeyohannes S, Perkins N, Goodwin W. Comparison of two formulations of alfaxalone in laboratory zebra fish (Danio rerio) for use in immersion anaesthesia. Oral abstract. 25th International Veterinary Emergency and Critical Care Society (IVECCS) Symposium, September 7th, 2019, Washington, D.C., USA. Session 171.
- Balko JA, Lindemann DA, Allender MC, Chinnadurai SK. Evaluation of the anesthetic and cardiorespiratory effects of intramuscular alfaxalone administration and isoflurane in budgerigars (Melopsittacus undulatus) and comparison with manual restraint. J Am Vet Med Assoc 2019;254(12):1427‑1435.
- Escalante GC, Balko JA, Chinnadurai, SK. Comparison of the sedative effects of alfaxalone and butorphanol‑midazolam administered intramuscularly in budgerigars (Melopsittacus undulatus). J Av Med Surg 2018;32(4):279‑285.
- Perrin KL, Nielsen JB, Thomsen AF, Bertelsen MF. Alfaxalone anesthesia in the Bengalese finch (Lonchura domestica). J Zoo Wildl Med 2017;48(4):1146‑1153.
- Villaverde‑Morcillo S, Benito J, García‑Sánchez R, Martín‑Jurado O, Gómez de Segura IA. Comparison of isoflurane and alfaxalone (Alfaxan) for the induction of anesthesia in flamingos (Phoenicopterus roseus) undergoing orthopedic surgery. J Zoo Wildl Med 2014;45(2):361‑366.
- Baldrey V. Assessment of alfaxalone as an anaesthetic induction agent in mute swans (Cygnus olor). Submitted in part fulfilment of the requirements for the Royal College of Veterinary Surgeons’ Diploma in Zoological Medicine 2014:1‑68.
- Gaudio E, Voltan L, De Benedictis GM. Alfaxalone anaesthesia in Lemur catta following dexmedetomidine‑butorphanol‑midazolam sedation. Vet Anaesth Analg 2018;45(3):351‑356.
- Passarelli L, Rosa MG, Gamberini M, Bakola S, Burman KJ, Fattori P, Galletti C. Cortical connections of area V6Av in the macaque: a visual‑input node to the eye/hand coordination system. J Neurosci 2011;31(5):1790‑1801.
- Bakker J, Uilenreef JJ, Pelt ER, Brok HP, Remarque EJ, Langermans JA. Comparison of three different sedative‑anaesthetic protocols (ketamine, ketamine‑medetomidine and alphaxalone) in common marmosets (Callithrix jacchus). BMC Vet Res 2013;9:1‑13.
- Ansel TV, Nour AK, Benavente‑Perez A. The effect of anesthesia on blood pressure measured noninvasively by using the tail‑cuff method in marmosets (Callithrix jacchus). J Am Assoc Lab Anim Sci 2016;55(5):594‑600.
- Curths C, Wichmann J, Dunker S, Windt H, Hoymann HG, Lauenstein HD, Hohlfeld J, Becker T, Kaup FJ, Braun A, Knauf S. Airway hyper‑responsiveness in lipopolysaccharide‑challenged common marmosets (Callithrix jacchus). Clin Sci 2014 126(2):155‑62.
- Sadoun A, Strelnikov K, Bonté E, Fonta C, Girard P. Cognitive impairment in a young marmoset reveals lateral ventriculomegaly and a mild hippocampal atrophy: a case report. Sci Rep 2015;5(16046):1‑11.
- Thomas AA, Leach MC, Flecknell PA. An alternative method of endotracheal intubation of common marmosets (Callithrix jacchus). Lab Anim 2012;46(1):71‑76.
- Tremoleda JL, Kerton A, Gsell W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI Res 2012;2(44):1‑23.
- Siriarchavatana P, Ayers JD, Kendall LV. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 2016;55(4):426‑30.
- Higuchi S, Yamada R, Hashimoto A, Miyoshi K, Yamashita K, Ohsugi T. Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn J Vet Res 2016;64(2):131‑139.
- Lau C, Ranasinghe MG, Shiels I, Keates H, Pasloske K, Bellingham MC. Plasma pharmacokinetics of alfaxalone after a single intraperitoneal or intravenous injection of Alfaxan® in rats. J Vet Pharmacol Ther 2013;36(5):516‑20.
- Reynolds PS, Song SK, Tamariz FJ, Barbee RW. Hypertension and vulnerability to hemorrhagic shock in a rat model. Shock 2015;43(2):148‑156.
- Arenillas M, Gomez de Segura IA. Anaesthetic effects of alfaxalone administered intraperitoneally alone or combined with dexmedetomidine and fentanyl in the rat. Lab Anim 2018;52(6):588‑598.
- White KL, Paine S, Harris J. A clinical evaluation of the pharmacokinetics and pharmacodynamics of intravenous alfaxalone in cyclodextrin in male and female rats following a loading dose and constant rate infusion. Vet Anaesth Analg 2017;44(4):865‑875.
- d’Ovidio D, Marino F, Noviello E, Lanaro E, Monticelli P, Adami C. Sedative effects of intramuscular alfaxalone in pet guinea pigs (Cavia porcellus). Vet Anaesth Analg 2018;45(2):183‑189.
- Doerning CM, Bradley MP, Lester PA, Nowland MH. Effects of subcutaneous alfaxalone alone and in combination with dexmedetomidine and buprenorphine in guinea pigs (Cavia porcellus). Vet Anaesth Analg 2018;45(5):658‑666.
- Bercier M, Langlois I, Dunn M, Hélie P, Burns P, Gara‑Boivin C. Cytological analysis of bronchoalveolar lavage fluid acquired by bronchoscopy in healthy ferrets: A pilot study. Can J Vet Res 2016;80(1):74‑80.
- Thas I. Acquired salivary mucoceles in two domestic ferrets (Mustela putorius furo). Vet Rec Case Rep 2014; 2:1‑8.
- Giral M, García‑Olmo DC, Gómez‑Juárez M, Gómez de Segura IA. Anaesthetic effects in the ferret of alfaxalone alone and in combination with medetomidine or tramadol: a pilot study. Lab Anim 2014;48(4):313‑320.
- Vogelnest L. Marsupialia (Marsupials). Fowler’s Zoo and Wild Animal Medicine. 2014; Volume 8, Chapter 33, p.260.
- McInnes LM, Gillett A, Ryan UM, Austen J, Campbell RS, Hanger J, Reid SA. Trypanosoma irwini n. sp (Sarcomastigophora: Trypanosomatidae) from the koala (Phascolarctos cinereus). Parasitology 2009;136(8):875‑885.
- McInnes LM, Hanger J, Simmons G, Reid SA, Ryan UM. Novel trypanosome Trypanosoma gilletti sp. (Euglenozoa: Trypanosomatidae) and the extension of the host range of Trypanosoma copemani to include the koala (Phascolarctos cinereus). Parasitology 2011;138(1):59‑70.
- McInnes LM, Gillett A, Hanger J, Reid SA, Ryan UM. The potential impact of native Australian trypanosome infections on the health of koalas (Phascolarctos cinereus). Parasitology 2011;138(7):873‑ 883.
- Markey B, Wan C, Hanger J, Phillips C, Timms P. Use of quantitative real‑time PCR to monitor the shedding and treatment of chlamydiae in the koala (Phascolarctos cinereus). Vet Microbiol 2007;120(3‑ 4):334‑342.
- Waugh C, Khan SA, Carver S, Hanger J, Loader J, Polkinghorne A, Beagley K, Timms P. A prototype recombinant‑protein based Chlamydia pecorum vaccine results in reduced chlamydial burden and less clinical disease in free‑ranging koalas (Phascolarctos cinereus). PLoS One 2016;11(1):1‑9.
- Liddle V, Naranjo C, Bernays M. Anterior chamber collapse syndrome in a koala. Aust Vet J 2014;92(5):179‑182.
- Bouts T, Karunaratna D, Berry K, Dodds J, Gasthuys F, Routh A, Taylor P. Evaluation of medetomidine‑ alfaxalone and medetomidine‑ketamine in semi‑free ranging Bennett’s wallabies (Macropus rufogriseus). J Zoo Wildl Med 2011;42(4):617‑622.
- del Álamo AM, Mandsager RE, Riebold TW, Payton ME. Evaluation of intravenous administration of alfaxalone, propofol, and ketamine‑diazepam for anesthesia in alpacas. Vet Anaesth Analg 2015;42(1):72‑82.
-
PRINCIPAL DISPLAY PANEL - 10 mL Vial
CIV
Alfaxan® Multidose
IDX
(alfaxalone) 10 mg/mL
For use as an injectable sedative and anesthetic in multiple non food-producing minor species.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
10 mL
NDC: 49480-003-01
Before using this product, read package insert for complete product information.
Distributed by Vedco, Inc.
Marketed by Jurox Inc. Phone 1-844-253-2926
Legally Marketed - MIF 900-031. Extra-label use is prohibited.
Store at controlled room temperature 20º to 25ºC (68º to 77º F) with excursions between 15° and 30°C (59° and 86°F).
Use within 56 days of first puncture.
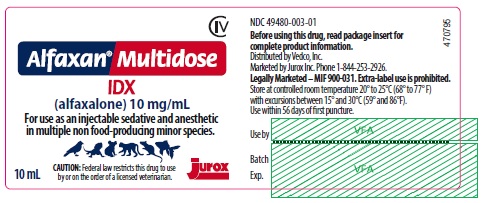
- PRINCIPAL DISPLAY PANEL - 10 mL Carton
-
Principal Display Panel - 20 mL Vial
CIV
Alfaxan® Multidose
IDX
(alfaxalone) 10 mg/mL
For use as an injectable sedative and anesthetic in multiple non food-producing minor species.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
20 mL
NDC: 49480-003-02
Before using this product, read package insert for complete product information.
Distributed by Vedco, Inc.
Marketed by Jurox Inc. Phone 1-844-253-2926
Legally Marketed - MIF 900-031. Extra-label use is prohibited.
Store at controlled room temperature 20º to 25ºC (68º to 77º F) with excursions between 15° and 30°C (59° and 86°F).
Use within 56 days of first puncture.
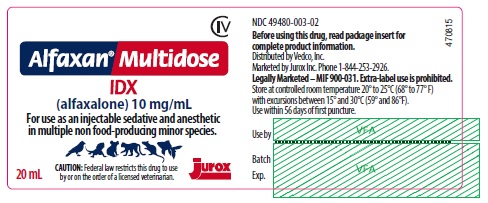
- Principal Display Panel - 20 mL Carton
-
INGREDIENTS AND APPEARANCE
ALFAXAN MULTIDOSE IDX
alfaxalone solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49480-003 Route of Administration INTRAVENOUS, INTRAMUSCULAR, SUBCUTANEOUS, INTRAPERITONEAL, TOPICAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALFAXALONE (UNII: BD07M97B2A) (ALFAXALONE - UNII:BD07M97B2A) ALFAXALONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYLBETADEX (0.58-0.68 MS) (UNII: 1I96OHX6EK) 80 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ALCOHOL (UNII: 3K9958V90M) 150 mg in 1 mL BENZETHONIUM CHLORIDE (UNII: PH41D05744) 0.2 mg in 1 mL CHLOROCRESOL (UNII: 36W53O7109) 1 mg in 1 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49480-003-01 1 in 1 CARTON 1 10 mL in 1 VIAL, GLASS 2 NDC: 49480-003-02 1 in 1 CARTON 2 20 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date legally marketed unapproved new animal drugs for minor species MIF900031 02/06/2020 Labeler - Jurox Pty. Limited (751438037) Establishment Name Address ID/FEI Business Operations Jurox Pty. Limited 751438037 api manufacture, analysis, label, manufacture, sterilize
Trademark Results [ALFAXAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALFAXAN 75661547 2548739 Live/Registered |
JUROX PTY LTD 1999-03-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.