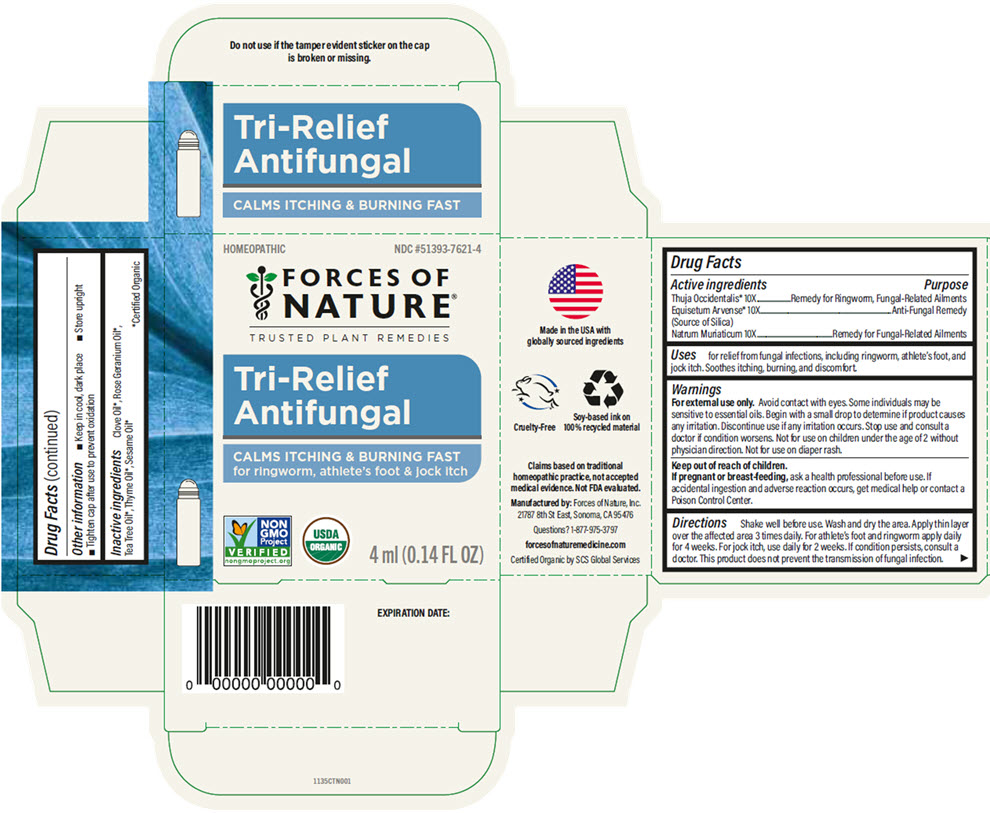
TRI-RELIEF ANTIFUNGAL- sodium chloride, silicon dioxide, and thuja occidentalis leafy twig solution/ drops
Tri-Relief Antifungal by
Drug Labeling and Warnings
Tri-Relief Antifungal by is a Homeopathic medication manufactured, distributed, or labeled by Forces of Nature. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only. Avoid contact with eyes. Some individuals may be sensitive to essential oils. Begin with a small drop to determine if product causes any irritation. Discontinue use if any irritation occurs. Stop use and consult a doctor if condition worsens. Not for use on children under the age of 2 without physician direction. Not for use on diaper rash.
-
Directions
Shake well before use. Wash and dry the area. Apply thin layer over the affected area 3 times daily. For athlete's foot and ringworm apply daily for 4 weeks. For jock itch, use daily for 2 weeks. If condition persists, consult a doctor. This product does not prevent the transmission of fungal infection.
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
TRI-RELIEF ANTIFUNGAL
sodium chloride, silicon dioxide, and thuja occidentalis leafy twig solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51393-7621 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 10 [hp_X] in 100 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 100 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 10 [hp_X] in 100 mL Inactive Ingredients Ingredient Name Strength SESAME OIL (UNII: QX10HYY4QV) TEA TREE OIL (UNII: VIF565UC2G) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) THYME OIL (UNII: 2UK410MY6B) CLOVE OIL (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51393-7621-4 1 in 1 CARTON 11/01/2025 1 4 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 11/01/2025 Labeler - Forces of Nature (050169130)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.