RIVAROXABAN tablet, film coated
RIVAROXABAN by
Drug Labeling and Warnings
RIVAROXABAN by is a Prescription medication manufactured, distributed, or labeled by Lupin Pharmaceuticals, Inc., LUPIN LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RIVAROXABAN TABLETS safely and effectively. See full prescribing information for RIVAROXABAN TABLETS.
RIVAROXABAN tablets, for oral use
Initial U.S. Approval: 2011
WARNING: (A) PREMATURE DISCONTINUATION OF RIVAROXABAN TABLETS INCREASES THE RISK OF THROMBOTIC EVENTS,
(B) SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete boxed warning
(A) Premature discontinuation of rivaroxaban tablets increases the risk of thrombotic events
Premature discontinuation of any oral anticoagulant, including rivaroxaban tablets, increases the risk of thrombotic events. To reduce this risk, consider coverage with another anticoagulant if rivaroxaban tablets are discontinued for a reason other than pathological bleeding or completion of a course of therapy. (2.2, 2.3, 5.1, 14.1)
(B) Spinal/epidural hematoma
Epidural or spinal hematomas have occurred in patients treated with rivaroxaban tablets who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. (5.2, 5.3, 6.2)
Monitor patients frequently for signs and symptoms of neurological impairment and if observed, treat urgently. Consider the benefits and risks before neuraxial intervention in patients who are or who need to be anticoagulated. (5.3)
RECENT MAJOR CHANGES
Warnings and Precautions (5.2) 06/2025
INDICATIONS AND USAGE
Rivaroxaban is a factor Xa inhibitor indicated:
- to reduce the risk of major cardiovascular events in patients with coronary artery disease (CAD) (1.7)
- to reduce the risk of major thrombotic vascular events in patients with peripheral artery disease (PAD), including patients after recent lower extremity revascularization due to symptomatic PAD (1.8)
DOSAGE AND ADMINISTRATION
- CAD or PAD: 2.5 mg orally twice daily with or without food, in combination with aspirin (75 to 100 mg) once daily (2.1).
DOSAGE FORMS AND STRENGTHS
Tablets: 2.5 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of Bleeding: Rivaroxaban tablets can cause serious and fatal bleeding. An agent to reverse the activity of rivaroxaban is available. (5.2)
- Pregnancy-Related Hemorrhage: Use rivaroxaban with caution in pregnant women due to the potential for obstetric hemorrhage and/or emergent delivery. (5.7, 8.1)
- Prosthetic Heart Valves: Rivaroxaban tablets use not recommended. (5.8)
- Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome: Rivaroxaban tablets use not recommended. (5.10)
ADVERSE REACTIONS
- The most common adverse reaction (>5 %) in adult patients was bleeding. (6.1)
- The most common adverse reactions (>10 %) in pediatric patients were bleeding, cough, vomiting, and gastroenteritis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: (A) PREMATURE DISCONTINUATION OF RIVAROXABAN TABLETS INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA
1 INDICATIONS AND USAGE
1.7 Reduction of Risk of Major Cardiovascular Events in Patients with Coronary Artery Disease (CAD)
1.8 Reduction of Risk of Major Thrombotic Vascular Events in Patients with Peripheral Artery Disease (PAD), Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
2.2 Recommended Dosage in Pediatric Patients
2.3 Switching to and from Rivaroxaban Tablets
2.4 Discontinuation for Surgery and Other Interventions
2.5 Missed Dose
2.6 Administration Options
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Thrombotic Events after Premature Discontinuation
5.2 Risk of Bleeding
5.3 Spinal/Epidural Anesthesia or Puncture
5.4 Use in Patients with Renal Impairment
5.5 Use in Patients with Hepatic Impairment
5.6 Use with P-gp and Strong CYP3A Inhibitors or Inducers
5.7 Risk of Pregnancy-Related Hemorrhage
5.8 Patients with Prosthetic Heart Valves
5.9 Acute PE in Hemodynamically Unstable Patients or Patients Who Require Thrombolysis or Pulmonary Embolectomy
5.10 Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 General Inhibition and Induction Properties
7.2 Drugs that Inhibit Cytochrome P450 3A Enzymes and Drug Transport Systems
7.3 Drugs that Induce Cytochrome P450 3A Enzymes and Drug Transport Systems
7.4 Anticoagulants and NSAIDs/Aspirin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 QT/QTc Prolongation
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.6 Reduction of Risk of Major Cardiovascular Events in Patients with CAD
14.7 Reduction of Risk of Major Thrombotic Vascular Events in Patients with PAD, Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: (A) PREMATURE DISCONTINUATION OF RIVAROXABAN TABLETS INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA
A. Premature Discontinuation of Rivaroxaban Tablets Increases the Risk of Thrombotic Events
Premature discontinuation of any oral anticoagulant, including rivaroxaban tablets, increases the risk of thrombotic events. If anticoagulation with rivaroxaban tablets are discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.3, 2.4 ), Warnings and Precautions (5.1), and Clinical Studies (14.1)].
B. Spinal/Epidural Hematoma
Epidural or spinal hematomas have occurred in patients treated with rivaroxaban tablets who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- a history of traumatic or repeated epidural or spinal punctures
- a history of spinal deformity or spinal surgery
- optimal timing between the administration of rivaroxaban tablets and neuraxial procedures is not known [see Warnings and Precautions (5.2, 5.3) and Adverse Reactions (6.2)].
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary [see Warnings and Precautions (5.3)].
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
1.7 Reduction of Risk of Major Cardiovascular Events in Patients with Coronary Artery Disease (CAD)
Rivaroxaban tablets, in combination with aspirin, are indicated to reduce the risk of major cardiovascular events (cardiovascular death, myocardial infarction, and stroke) in adult patients with coronary artery disease.
1.8 Reduction of Risk of Major Thrombotic Vascular Events in Patients with Peripheral Artery Disease (PAD), Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
Rivaroxaban tablets, in combination with aspirin, are indicated to reduce the risk of major thrombotic vascular events (myocardial infarction, ischemic stroke, acute limb ischemia, and major amputation of a vascular etiology) in adult patients with PAD, including patients who have recently undergone a lower extremity revascularization procedure due to symptomatic PAD.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
Table 1: Recommended Dosage in Adults - * Calculate CrCl based on actual weight. [See Warnings and Precautions (5.4) and Use in Specific Populations (8.6)].
- † See Clinical Pharmacology (12.3)
Indication
Renal Considerations*
Dosage
Food/Timing†
Reduction of Risk of Major Cardiovascular Events (CV Death, MI, and Stroke) in CAD
No dose adjustment needed based on CrCl
2.5 mg twice daily , plus aspirin (75 to 100 mg) once daily
Take with or without food
Reduction of Risk of Major Thrombotic Vascular Events in PAD, Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
No dose adjustment needed based on CrCl
2.5 mg twice daily , plus aspirin (75 to 100 mg) once daily.
When starting therapy after a successful lower extremity revascularization procedure, initiate once hemostasis has been established.
Take with or without food
2.2 Recommended Dosage in Pediatric Patients
Rivaroxaban 2.5 mg tablets are not recommended for use in pediatric patients [see Use in Specific Populations (8.4)].
2.3 Switching to and from Rivaroxaban Tablets
Switching from Warfarin to Rivaroxaban Tablets
When switching patients from warfarin to rivaroxaban tablets, discontinue warfarin and start rivaroxaban tablets as soon as the International Normalized Ratio (INR) is below 3 in adults and below 2.5 in pediatric patients to avoid periods of inadequate anticoagulation.
Switching from Rivaroxaban Tablets to Warfarin
- Adults:
No clinical trial data are available to guide converting patients from rivaroxaban tablets to warfarin. Rivaroxaban tablet affects INR, so INR measurements made during coadministration with warfarin may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue rivaroxaban tablets and begin both a parenteral anticoagulant and warfarin at the time the next dose of rivaroxaban tablets would have been taken.
Once rivaroxaban is discontinued, INR testing may be done reliably 24 hours after the last dose.
Switching from Rivaroxaban Tablets to Anticoagulants other than Warfarin
Patients currently taking rivaroxaban tablets and transitioning to an anticoagulant with rapid onset, discontinue rivaroxaban tablet and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next rivaroxaban tablets dose would have been taken [see Drug Interactions (7.4)].
Switching from Anticoagulants other than Warfarin to Rivaroxaban tablets
Patients currently receiving an anticoagulant other than warfarin, start rivaroxaban tablets 0 to 2 hours prior to the next scheduled administration of the drug (e.g., low molecular weight heparin or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start rivaroxaban tablets at the same time.
2.4 Discontinuation for Surgery and Other Interventions
If anticoagulation must be discontinued to reduce the risk of bleeding with surgical or other procedures, rivaroxaban tablets should be stopped at least 24 hours before the procedure to reduce the risk of bleeding [see Warnings and Precautions (5.2)]. In deciding whether a procedure should be delayed until 24 hours after the last dose of rivaroxaban tablets, the increased risk of bleeding should be weighed against the urgency of intervention. Rivaroxaban tablets should be restarted after the surgical or other procedures as soon as adequate hemostasis has been established, noting that the time to onset of therapeutic effect is short [see Warnings and Precautions (5.1)]. If oral medication cannot be taken during or after surgical intervention, consider administering a parenteral anticoagulant.
2.5 Missed Dose
- For patients receiving 2.5 mg twice daily: if a dose is missed, the patient should take a single 2.5 mg rivaroxaban tablet dose as recommended at the next scheduled time.
On the following day, the patient should continue with their regular regimen.
2.6 Administration Options
For adult patients who are unable to swallow whole tablets, rivaroxaban tablets (all strengths) may be crushed and mixed with applesauce immediately prior to use and administered orally. Administration with food is not required for the 2.5 mg tablets [see Clinical Pharmacology (12.3)].
Administration of Rivaroxaban Tablets via Nasogastric (NG) Tube or Gastric Feeding Tube:
After confirming gastric placement of the tube, rivaroxaban tablets (all strengths) may be crushed and suspended in 50 mL of water and administered via an NG tube or gastric feeding tube. Since rivaroxaban absorption is dependent on the site of drug release, avoid administration of rivaroxaban tablets distal to the stomach which can result in reduced absorption and thereby, reduced drug exposure. Enteral feeding is not required following administration of the 2.5 mg tablets [see Clinical Pharmacology (12.3)].
Crushed rivaroxaban tablets (all strengths) are stable in water and in applesauce for up to 4 hours. An in vitro compatibility study indicated that there is no adsorption of rivaroxaban from a water suspension of a crushed rivaroxaban tablets to PVC or silicone nasogastric (NG) tubing.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Thrombotic Events after Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including rivaroxaban tablets, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from rivaroxaban tablets to warfarin in clinical trials in atrial fibrillation patients. If rivaroxaban tablets are discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.3,2.4) and Clinical Studies (14.1)].
5.2 Risk of Bleeding
Rivaroxaban tablets increase the risk of bleeding, including in any organ, and can cause serious or fatal bleeding. In deciding whether to prescribe rivaroxaban tablets to patients at increased risk of bleeding, the risk of thrombotic events should be weighed against the risk of bleeding.
Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement. Discontinue rivaroxaban tablets in patients with active pathological hemorrhage. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
Concomitant use of other drugs that impair hemostasis increases the risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, dual antiplatelet therapy, other antithrombotic agents, fibrinolytic therapy, non-steroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.4)], selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors.
Concomitant use of drugs that are known combined P-gp and strong CYP3A inhibitors increases rivaroxaban exposure and may increase bleeding risk [see Drug Interactions (7.2)].
Reversal of Anticoagulant Effect
An agent to reverse the anti-factor Xa activity of rivaroxaban is available. Because of high plasma protein binding, rivaroxaban is not dialyzable [see Clinical Pharmacology (12.3)]. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban. Use of procoagulant reversal agents, such as prothrombin complex concentrate (PCC), activated prothrombin complex concentrate or recombinant factor VIIa, may be considered but has not been evaluated in clinical efficacy and safety studies. Monitoring for the anticoagulation effect of rivaroxaban using a clotting test (PT, INR or aPTT) or anti-factor Xa (FXa) activity is not recommended.
5.3 Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis [see Boxed Warning].
To reduce the potential risk of bleeding associated with the concurrent use of rivaroxaban tablets and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of rivaroxaban tablets [see Clinical Pharmacology (12.3)]. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of rivaroxaban tablets is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
An indwelling epidural or intrathecal catheter should not be removed before at least 2 half-lives have elapsed (i.e., 18 hours in young patients aged 20 to 45 years and 26 hours in elderly patients aged 60 to 76 years), after the last administration of rivaroxaban tablets [see Clinical Pharmacology (12.3)]. The next rivaroxaban tablets dose should not be administered earlier than 6 hours after the removal of the catheter. If traumatic puncture occurs, delay the administration of rivaroxaban tablets for 24 hours.
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
5.4 Use in Patients with Renal Impairment
Discontinue rivaroxaban tablets in patients who develop acute renal failure while on treatment [see Use in Specific Populations (8.6)].
5.5 Use in Patients with Hepatic Impairment
No clinical data are available for adult patients with severe hepatic impairment.
Avoid use of rivaroxaban tablets in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy since drug exposure and bleeding risk may be increased [see Use in Specific Populations (8.7)].
No clinical data are available in pediatric patients with hepatic impairment.
5.6 Use with P-gp and Strong CYP3A Inhibitors or Inducers
Avoid concomitant use of rivaroxaban tablets with known combined P-gp and strong CYP3A inhibitors [see Drug Interactions (7.2)].
Avoid concomitant use of rivaroxaban tablets with drugs that are known combined P-gp and strong CYP3A inducers [see Drug Interactions (7.3)].
5.7 Risk of Pregnancy-Related Hemorrhage
In pregnant women, rivaroxaban tablets should be used only if the potential benefit justifies the potential risk to the mother and fetus. Rivaroxaban tablets dosing in pregnancy has not been studied. The anticoagulant effect of rivaroxaban tablets cannot be monitored with standard laboratory testing. Promptly evaluate any signs or symptoms suggesting blood loss (e.g., a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress) [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
5.8 Patients with Prosthetic Heart Valves
On the basis of the GALILEO study, use of rivaroxaban tablets is not recommended in patients who have had transcatheter aortic valve replacement (TAVR) because patients randomized to rivaroxaban tablets experienced higher rates of death and bleeding compared to those randomized to an anti-platelet regimen. The safety and efficacy of rivaroxaban tablets have not been studied in patients with other prosthetic heart valves or other valve procedures. Use of rivaroxaban tablets is not recommended in patients with prosthetic heart valves.
5.9 Acute PE in Hemodynamically Unstable Patients or Patients Who Require Thrombolysis or Pulmonary Embolectomy
Initiation of rivaroxaban tablets are not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy.
5.10 Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome
Direct-acting oral anticoagulants (DOACs), including rivaroxaban tablets, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Increased Risk of Stroke After Discontinuation in Nonvalvular Atrial Fibrillation[see Boxed Warning and Warnings and Precautions (5.1)]
- Bleeding Risk [see Warnings and Precautions (5.2, 5.4, 5.5, 5.6, 5.7)]
- Spinal/Epidural Hematoma [see Boxed Warning and Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical development for the approved indications, 34, 947 adult patients were exposed to rivaroxaban tablets.
Hemorrhage
The most common adverse reactions with rivaroxaban tablets were bleeding complications [see Warnings and Precautions (5.2)].
Reduction of Risk of Major Cardiovascular Events in Patients with CAD:
In the COMPASS trial overall, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 2.7 % for rivaroxaban tablets 2.5 mg twice daily vs. 1.2 % for placebo on background therapy for all patients with aspirin 100 mg once daily. The incidences of important bleeding events in the CAD and PAD populations in COMPASS were similar.
Table 10 shows the number of patients experiencing various types of major bleeding events in the COMPASS trial.
Table 10: Major Bleeding Events in COMPASS -On Treatment Plus 2 Days* *Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment in the safety analysis set in COMPASS patients.
†Treatment schedule: rivaroxaban tablets 2.5 mg twice daily or placebo. All patients received background therapy with aspirin 100 mg once daily
‡Defined as i) fatal bleeding, or ii) symptomatic bleeding in a critical area or organ, such as intraarticular, intramuscular with compartment syndrome, intraspinal, intracranial, intraocular, respiratory, pericardial, liver, pancreas, retroperitoneal, adrenal gland or kidney; or iii) bleeding into the surgical site requiring reoperation, or iv) bleeding leading to hospitalization.
CI:confidence interval; HR: hazard ratio; ISTH: International Society on Thrombosis and Hemostasis
Parameter
Rivaroxaban Tablets† N=9134
n (%/year)
Placebo† N=9107
n (%/year)
Rivaroxaban Tablets plus Placebo
HR (95 % CI)
Modified ISTH Major Bleeding‡
263 (1.6)
144 (0.9)
1.8 (1.5, 2.3)
- Fatal bleeding event
Intracranial hemorrhage (ICH)
Non-intracranial
12 (<0.1)
6 (<0.1)
6 (<0.1)
8 (<0.1)
3 (<0.1)
5 (<0.1)
1.5 (0.6, 3.7)
2.(0.5, 8)
1.2 (0.4, 4)
- Symptomatic bleeding in critical organ (non-fatal)
- ICH (fatal and non-fatal)
Hemorrhagic Stroke
Other ICH
58 (0.3)
23 (0.1)
18 (0.1)
6 (<0.1)
43 (0.3)
21 (0.1)
13 (<0.1)
9 (<0.1)
1.4 (0.9, 2)
1.1 (0.6, 2)
1.4 (0.7, 2.8)
0.7 (0.2, 1.9)
- Bleeding into the surgical site requiring reoperation (non-fatal, not in critical organ)
7 (<0.1)
6 (<0.1)
1.2 (0.4, 3.5)
- Bleeding leading to hospitalization (non-fatal, not in critical organ, not requiring reoperation)
188 (1.1)
91 (0.5)
2.1 (1.6, 2.7)
Major GI bleeding
117 (0.7)
49 (0.3)
2.4 (1.7, 3.4)
Reduction of Risk of Major Thrombotic Vascular Events in Patients with Peripheral Artery Disease (PAD), Including Patients after Lower Extremity Revascularization due to Symptomatic PAD:
The incidence of premature permanent discontinuation due to bleeding events for rivaroxaban tablets 2.5 mg twice daily vs. placebo on background therapy with aspirin 100 mg once daily in VOYAGER was 4.1 % vs. 1.6 % and in COMPASS PAD was 2.7 % vs. 1.3 %, respectively.
Table 11 shows the number of patients experiencing various types of TIMI (Thrombolysis in Myocardial Infarction) major bleeding events in the VOYAGER trial. The most common site of bleeding was gastrointestinal.
Table 11: Major Bleeding Events* in VOYAGER-On Treatment Plus 2 Days * Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories.
† Treatment schedule: rivaroxaban tablets 2.5 mg twice daily or placebo. All patients received background therapy with aspirin 100 mg once daily.
CABG: Coronary artery bypass graft; CI: confidence interval; HR: hazard ratio; TIMI: Thrombolysis in Myocardial Infarction Bleeding Criteria
Rivaroxaban Tablets† N=3256
Placebo†
N=3248
Rivaroxaban Tablets vs. Placebo
HR (95 % CI)
Parameter
n (%)
Event rate %/year
n (%)
Event rate %/year
TIMI Major Bleeding
(CABG/non-CABG)
62 (1.9)
0.96
44 (1.4)
0.67
1.4 (1, 2.1)
Fatal bleeding
6 (0.2)
0.09
6 (0.2)
0.09
1 (0.3, 3.2)
Intracranial bleeding
13 (0.4)
0.20
17 (0.5)
0.26
0.8 (0.4, 1.6)
Clinically overt signs of hemorrhage associated with a drop in hemoglobin of ≥5 g/dL or drop in hematocrit of ≥15 %
46 (1.4)
0.71
24 (0.7)
0.36
1.9 (1.2, 3.2)
Non-hemorrhagic adverse reactions reported in ≥1 % of rivaroxaban-treated patients in the EINSTEIN DVT and EINSTEIN PE studies are shown in Table 12.
Table 12: Other Adverse Reactions* Reported by ≥1 % of Rivaroxaban-Treated Patients in EINSTEIN DVT and EINSTEIN PE Studies - * Adverse reaction with Relative Risk >1.5 for rivaroxaban tablets versus comparator
Body System
Adverse Reaction
EINSTEIN DVT Study
Rivaroxaban Tablets 20 mg
N=1718
n (%)
Enoxaparin/VKA
N=1711
n (%)
Gastrointestinal disorders
Abdominal pain
46 (2.7)
25 (1.5)
General disorders and administration site conditions
Fatigue
24 (1.4)
15 (0.9)
Musculoskeletal and connective tissue disorders
Back pain
50 (2.9)
31 (1.8)
Muscle spasm
23 (1.3)
13 (0.8)
Nervous system disorders
Dizziness
38 (2.2)
22 (1.3)
Psychiatric disorders
Anxiety
24 (1.4)
11 (0.6)
Depression
20 (1.2)
10 (0.6)
Insomnia
28 (1.6)
18 (1.1)
EINSTEIN PE Study
Rivaroxaban Tablets 20 mg
N=2412
n (%)
Enoxaparin/VKA
N=2405
n (%)
Skin and subcutaneous tissue disorders
Pruritus
53 (2.2)
27 (1.1)
Non-hemorrhagic adverse reactions reported in ≥1 % of rivaroxaban-treated patients in RECORD 1 to 3 studies are shown in Table 13.
Table 13: Other Adverse Drug Reactions*Reported by ≥1 % of Rivaroxaban -Treated Patients in RECORD 1 to 3 Studies * Adverse reaction occurring any time following the first dose of double-blind medication, which may have been prior to administration of active drug, until two days after the last dose of double-blind study medication
† Includes the placebo-controlled period of RECORD 2, enoxaparin dosing was 40 mg once daily (RECORD 1 to 3)
Body System
Adverse Reaction
Rivaroxaban Tablets 10 mg
N=4487
n (%)
Enoxaparin†
N=4524
n (%)
Injury, poisoning and procedural complications
Wound secretion
125 (2.8)
89 (2)
Musculoskeletal and connective tissue disorders
Pain in extremity
74 (1.7)
55 (1.2)
Muscle spasm
52 (1.2)
32 (0.7)
Nervous system disorders
Syncope
55 (1.2)
32 (0.7)
Skin and subcutaneous tissue disorders
Pruritus
96 (2.1)
79 (1.8)
Blister
63 (1.4)
40 (0.9)
Non-bleeding adverse reactions reported in ≥5 % of rivaroxaban-treated patients are shown in Table 17.
Table 17: Other Adverse Reactions*Reported by ≥5 % of Rivaroxaban -Treated Patients in UNIVERSE Study (Part B) * Adverse reaction with Relative Risk >1.5 for rivaroxaban tablets versus aspirin.
† The following terms were combined:
Gastroenteritis: gastroenteritis, gastroenteritis viral
Rash: rash, rash maculo-papular, viral rash
Adverse Reaction
Rivaroxaban Tablets
N=64
n (%)
Aspirin N=34
n (%)
Cough
10 (15.6)
3 (8.8)
Vomiting
9 (14.1)
3 (8.8)
Gastroenteritis†
8 (12.5)
1 (2.9)
Rash†
6 (9.4)
2 (5.9)
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of rivaroxaban tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: agranulocytosis, thrombocytopenia
Hepatobiliary Disorders: jaundice, cholestasis, hepatitis (including hepatocellular injury)
Immune System Disorders: hypersensitivity, anaphylactic reaction, anaphylactic shock, angioedema
Nervous System Disorders: hemiparesis
Renal Disorders: Anticoagulant-related nephropathy
Respiratory, Thoracic and Mediastinal Disorders: Eosinophilic pneumonia
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms (DRESS)
Injury, poisoning and procedural complications: Atraumatic splenic rupture
-
7 DRUG INTERACTIONS
7.1 General Inhibition and Induction Properties
Rivaroxaban is a substrate of CYP3A4/5, CYP2J2, and the P-gp and ATP-binding cassette G2 (ABCG2) transporters. Combined P-gp and strong CYP3A inhibitors increase exposure to rivaroxaban and may increase the risk of bleeding. Combined P-gp and strong CYP3A inducers decrease exposure to rivaroxaban and may increase the risk of thromboembolic events.
7.2 Drugs that Inhibit Cytochrome P450 3A Enzymes and Drug Transport Systems
Interaction with Combined P-gp and Strong CYP3A Inhibitors
Avoid concomitant administration of rivaroxaban tablets with known combined P-gp and strong CYP3A inhibitors (e.g., ketoconazole and ritonavir) [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
Although clarithromycin is a combined P-gp and strong CYP3A inhibitor, pharmacokinetic data suggests that no precautions are necessary with concomitant administration with rivaroxaban tablets as the change in exposure is unlikely to affect the bleeding risk [see Clinical Pharmacology (12.3)].
Interaction with Combined P-gp and Moderate CYP3A Inhibitors in Patients with Renal Impairment
Rivaroxaban tablets should not be used in patients with CrCl 15 to <80 mL/min who are receiving concomitant combined P-gp and moderate CYP3A inhibitors (e.g., erythromycin) unless the potential benefit justifies the potential risk [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
7.3 Drugs that Induce Cytochrome P450 3A Enzymes and Drug Transport Systems
Avoid concomitant use of rivaroxaban tablets with drugs that are combined P-gp and strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort) [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.4 Anticoagulants and NSAIDs/Aspirin
Coadministration of enoxaparin, warfarin, aspirin, clopidogrel and chronic NSAID use may increase the risk of bleeding [see Clinical Pharmacology (12.3)].
Avoid concurrent use of rivaroxaban tablets with other anticoagulants due to increased bleeding risk unless benefit outweighs risk. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
The limited available data on rivaroxaban tablets in pregnant women are insufficient to inform a drug- associated risk of adverse developmental outcomes. Use rivaroxaban tablets with caution in pregnant patients because of the potential for pregnancy related hemorrhage and/or emergent delivery. The anticoagulant effect of rivaroxaban tablets cannot be reliably monitored with standard laboratory testing. Consider the benefits and risks of rivaroxaban tablets for the mother and possible risks to the fetus when prescribing rivaroxaban tablets to a pregnant woman [see Warnings and Precautions (5.2, 5.7)].
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 % and 15 to 20 %, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk:
Pregnancy is a risk factor for venous thromboembolism and that risk is increased in women with inherited or acquired thrombophilias. Pregnant women with thromboembolic disease have an increased risk of maternal complications including pre-eclampsia. Maternal thromboembolic disease increases the risk for intrauterine growth restriction, placental abruption and early and late pregnancy loss.
Fetal/Neonatal Adverse Reactions :
Based on the pharmacologic activity of Factor Xa inhibitors and the potential to cross the placenta, bleeding may occur at any site in the fetus and/or neonate.
Labor or Delivery :
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding and this risk may be increased during labor or delivery [see Warnings and Precautions (5.7)]. The risk of bleeding should be balanced with the risk of thrombotic events when considering the use of rivaroxaban tablets in this setting.
Data
Human Data :
There are no adequate or well-controlled studies of rivaroxaban tablets in pregnant women, and dosing for pregnant women has not been established. Post-marketing experience is currently insufficient to determine a rivaroxaban-associated risk for major birth defects or miscarriage. In an in vitro placenta perfusion model, unbound rivaroxaban was rapidly transferred across the human placenta.
Animal Data:
Rivaroxaban crosses the placenta in animals. Rivaroxaban increased fetal toxicity (increased resorptions, decreased number of live fetuses, and decreased fetal body weight) when pregnant rabbits were given oral doses of ≥10 mg/kg rivaroxaban during the period of organogenesis. This dose corresponds to about 4 times the human exposure of unbound drug, based on AUC comparisons at the highest recommended human dose of 20 mg/day. Fetal body weights decreased when pregnant rats were given oral doses of 120 mg/kg during the period of organogenesis. This dose corresponds to about 14 times the human exposure of unbound drug. In rats, peripartal maternal bleeding and maternal and fetal death occurred at the rivaroxaban dose of 40 mg/kg (about 6 times maximum human exposure of the unbound drug at the human dose of 20 mg/day).
8.2 Lactation
Rivaroxaban has been detected in human milk. There are insufficient data to determine the effects of rivaroxaban on the breastfed child or on milk production. Rivaroxaban and/or its metabolites were present in the milk of rats. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for rivaroxaban tablets and any potential adverse effects on the breastfed infant from rivaroxaban tablets or from the underlying maternal condition (see Data).
Data
Animal Data:
Following a single oral administration of 3 mg/kg of radioactive [14C]-rivaroxaban to lactating rats between Day 8 to 10 postpartum, the concentration of total radioactivity was determined in milk samples collected up to 32 hours post-dose. The estimated amount of radioactivity excreted with milk within 32 hours after administration was 2.1 % of the maternal dose.
8.3 Females and Males of Reproductive Potential
Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician.
The risk of clinically significant uterine bleeding, potentially requiring gynecological surgical interventions, identified with oral anticoagulants including rivaroxaban tablets should be assessed in females of reproductive potential and those with abnormal uterine bleeding.
8.4 Pediatric Use
For the rivaroxaban 2.5 mg tablets, there are no safety, efficacy, pharmacokinetic and pharmacodynamic data to support the use in pediatric patients. Therefore, rivaroxaban tablets 2.5 mg tablets are not recommended for use in pediatric patients.
8.5 Geriatric Use
Of the total number of adult patients in clinical trials for the approved indications of rivaroxaban tablets (N=64,943 patients), 64 percent were 65 years and over, with 27 percent 75 years and over. In clinical trials the efficacy of rivaroxaban tablets in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.6 Renal Impairment
In pharmacokinetic studies, compared to healthy adult subjects with normal creatinine clearance, rivaroxaban exposure increased by approximately 44 to 64 % in adult subjects with renal impairment. Increases in pharmacodynamic effects were also observed [see Clinical Pharmacology (12.3)].
Reduction of Risk of Major Cardiovascular Events in Patients with CAD and Reduction of Risk of Major Thrombotic Vascular Events in Patients with PAD, Including Patients After Recent Lower Extremity Revascularization due to Symptomatic PAD
Patients with Chronic Kidney Disease not on Dialysis:
Patients with a CrCl <15 mL/min at screening were excluded from COMPASS and VOYAGER, and limited data are available for patients with a CrCl of 15 to 30 mL/min. In patients with CrCl <30 mL/min, a dose of 2.5 mg rivaroxaban tablets twice daily is expected to give an exposure similar to that in patients with moderate renal impairment (CrCl 30 to <50 mL/min) [see Clinical Pharmacology (12.3)], whose efficacy and safety outcomes were similar to those with preserved renal function.
Patients with End-Stage Renal Disease on Dialysis:
No clinical outcome data is available for the use of rivaroxaban tablets with aspirin in patients with ESRD on dialysis since these patients were not enrolled in COMPASS or VOYAGER. In patients with ESRD maintained on intermittent hemodialysis, administration of rivaroxaban tablets 2.5 mg twice daily will result in concentrations of rivaroxaban and pharmacodynamic activity similar to those observed in moderate renal impaired patients in the COMPASS study [see Clinical Pharmacology (12.2, 12.3)]. It is not known whether these concentrations will lead to similar CV risk reduction and bleeding risk in patients with ESRD on dialysis as was seen in COMPASS.
8.7 Hepatic Impairment
In a pharmacokinetic study, compared to healthy adult subjects with normal liver function, AUC increases of 127 % were observed in adult subjects with moderate hepatic impairment (Child-Pugh B).
The safety or PK of rivaroxaban tablets in patients with severe hepatic impairment (Child-Pugh C) has not been evaluated [see Clinical Pharmacology (12.3)].
Avoid the use of rivaroxaban tablets in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy.
No clinical data are available in pediatric patients with hepatic impairment.
-
10 OVERDOSAGE
Overdose of rivaroxaban tablets may lead to hemorrhage. Discontinue rivaroxaban tablets and initiate appropriate therapy if bleeding complications associated with overdosage occur. Rivaroxaban systemic exposure is not further increased at single doses >50 mg due to limited absorption. The use of activated charcoal to reduce absorption in case of rivaroxaban tablets overdose may be considered. Due to the high plasma protein binding, rivaroxaban is not dialyzable [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. Partial reversal of laboratory anticoagulation parameters may be achieved with use of plasma products. An agent to reverse the anti-factor Xa activity of rivaroxaban is available.
-
11 DESCRIPTION
Rivaroxaban, a factor Xa (FXa) inhibitor, is the active ingredient in rivaroxaban tablets with the chemical name 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl) phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide. The molecular formula of rivaroxaban is C19H18ClN3O5S and the molecular weight is 435.88. The structural formula is:
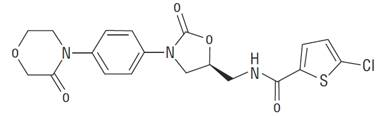
Rivaroxaban is a pure (S)-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. Rivaroxaban is only slightly soluble in organic solvents (e.g., acetone, polyethylene glycol 400) and is practically insoluble in water and aqueous media.
Each rivaroxaban tablet, USP contains 2.5 mg of rivaroxaban. The inactive ingredients of rivaroxaban tablets are: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. Additionally, the proprietary film coating mixture used for rivaroxaban tablets, USP 2.5 mg are Opadry®Yellow containing hypromellose, iron oxide yellow, macrogol / PEG and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rivaroxaban is a selective inhibitor of FXa. It does not require a cofactor (such as Anti-thrombin III) for activity. Rivaroxaban inhibits free FXa and prothrombinase activity. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation.
12.2 Pharmacodynamics
Rivaroxaban produces dose-dependent inhibition of FXa activity. Clotting tests, such as prothrombin time (PT), activated partial thromboplastin time (aPTT) and HepTest®, are also prolonged dose-dependently. In children treated with rivaroxaban, the correlation between anti-factor Xa to plasma concentrations is linear with a slope close to 1.
Monitoring for anticoagulation effect of rivaroxaban using anti-FXa activity or a clotting test is not recommended.
Specific Populations
Renal Impairment:
The relationship between systemic exposure and pharmacodynamic activity of rivaroxaban was altered in adult subjects with renal impairment relative to healthy control subjects [see Use in Specific Populations (8.6)].
Table 18: Percentage Increase in Rivaroxaban PK and PD Measures in Adult Subjects with Renal Impairment Relative to Healthy Subjects from Clinical Pharmacology Studies *Separate stand-alone study.
PT = Prothrombin time; FXa = Coagulation factor Xa; AUC = Area under the plasma concentration-time curve; AUEC = Area under the effect-time curve
Measure
Parameter
Creatinine Clearance (mL/min)
50 to79
30 to 49
15 to 29
ESRD (on dialysis)*
ESRD (post-dialysis)*
Exposure
AUC
44
52
64
47
56
FXa Inhibition
AUEC
50
86
100
49
33
PT Prolongation
AUEC
33
116
144
112
158
Anti-Factor Xa activity was similar in adult subjects with normal hepatic function and in mild hepatic impairment (Child-Pugh A class). There is no clear understanding of the impact of hepatic impairment beyond this degree on the coagulation cascade and its relationship to efficacy and safety.
12.3 Pharmacokinetics
The absolute bioavailability of rivaroxaban is dose-dependent. For the 2.5 mg and 10 mg dose, it is estimated to be 80 % to 100 % and is not affected by food. Rivaroxaban 2.5 mg and 10 mg tablets can be taken with or without food. Rivaroxaban tablets 20 mg administered in the fasted state has an absolute bioavailability of approximately 66 %. Coadministration of rivaroxaban tablets with food increases the bioavailability of the 20 mg dose (mean AUC and Cmax increasing by 39 % and 76 % respectively with food). Rivaroxaban 15 mg and 20 mg tablets should be taken with food [see Dosage and Administration (2.1)].
The maximum concentrations (Cmax) of rivaroxaban appear 2 to 4 hours after tablet intake. The pharmacokinetics of rivaroxaban were not affected by drugs altering gastric pH. Coadministration of rivaroxaban tablets (30 mg single dose) with the H2-receptor antagonist ranitidine (150 mg twice daily), the antacid aluminum hydroxide/magnesium hydroxide (10 mL) or rivaroxaban tablets (20 mg single dose) with the PPI omeprazole (40 mg once daily) did not show an effect on the bioavailability and exposure of rivaroxaban (see Figure 3).
Absorption of rivaroxaban is dependent on the site of drug release in the GI tract. A 29 % and 56 % decrease in AUC and Cmax compared to tablet was reported when rivaroxaban granulate is released in the proximal small intestine. Exposure is further reduced when drug is released in the distal small intestine, or ascending colon. Avoid administration of rivaroxaban distal to the stomach which can result in reduced absorption and related drug exposure.
In a study with 44 healthy subjects, both mean AUC and Cmax values for 20 mg rivaroxaban administered orally as a crushed tablet mixed in applesauce were comparable to that after the whole tablet. However, for the crushed tablet suspended in water and administered via an NG tube followed by a liquid meal, only mean AUC was comparable to that after the whole tablet, and Cmax was 18 % lower.
Distribution
Protein binding of rivaroxaban in human plasma is approximately 92 % to 95 %, with albumin being the main binding component. The steady-state volume of distribution in healthy subjects is approximately 50 L.
Metabolism
Approximately 51 % of an orally administered [14C]-rivaroxaban dose was recovered as inactive metabolites in urine (30 %) and feces (21 %). Oxidative degradation catalyzed by CYP3A4/5 and CYP2J2 and hydrolysis are the major sites of biotransformation. Unchanged rivaroxaban was the predominant moiety in plasma with no major or active circulating metabolites.
Excretion
In a Phase 1 study, following the administration of [14C]-rivaroxaban, approximately one-third (36 %) was recovered as unchanged drug in the urine and 7 % was recovered as unchanged drug in feces. Unchanged drug is excreted into urine, mainly via active tubular secretion and to a lesser extent via glomerular filtration (approximate 5:1 ratio). Rivaroxaban is a substrate of the efflux transporter proteins P-gp and ABCG2 (also abbreviated BCRP). Rivaroxaban's affinity for influx transporter proteins is unknown.
Rivaroxaban is a low-clearance drug, with a systemic clearance of approximately 10 L/hr in healthy volunteers following intravenous administration. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
Specific Populations
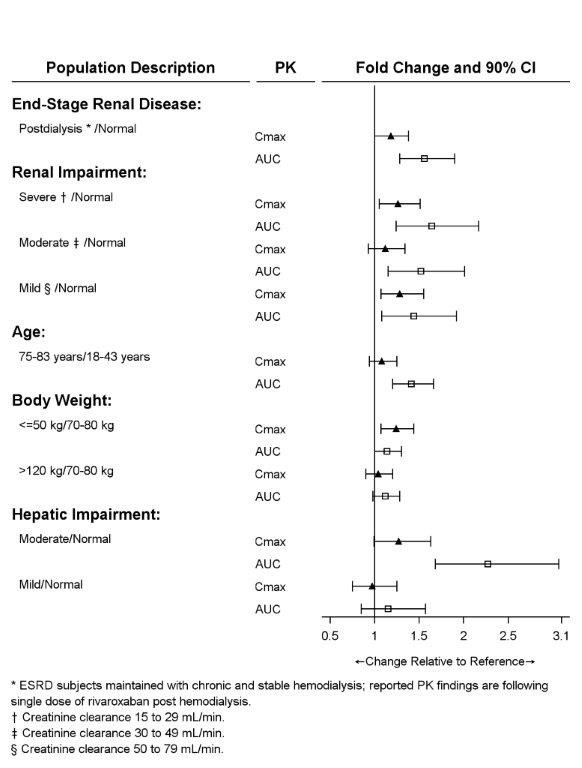
The effects of level of renal impairment, age, body weight, and level of hepatic impairment on the pharmacokinetics of rivaroxaban are summarized in Figure 2.
Figure 2: Effect of Specific Adult Populations on the Pharmacokinetics of Rivaroxaban
[See Dosage and Administration (2.1)].
Gender :
Gender did not influence the pharmacokinetics or pharmacodynamics of rivaroxaban tablets.
Race:
Healthy Japanese subjects were found to have 20 to 40 % on average higher exposures compared to other ethnicities including Chinese. However, these differences in exposure are reduced when values are corrected for body weight.
Elderly :
The terminal elimination half-life is 11 to 13 hours in the elderly subjects aged 60 to 76 years [see Use in Specific Populations (8.5)].
Renal Impairment:
The safety and pharmacokinetics of single-dose rivaroxaban tablets (10 mg) were evaluated in a study in healthy subjects [CrCl ≥80 mL/min (n=8)] and in subjects with varying degrees of renal impairment (see Figure 2). Compared to healthy subjects with normal creatinine clearance, rivaroxaban exposure increased in subjects with renal impairment. Increases in pharmacodynamic effects were also observed [see Use in Specific Populations (8.6)].
Hemodialysis in ESRD subjects
Systemic exposure to rivaroxaban administered as a single 15 mg dose in ESRD subjects dosed 3 hours after the completion of a 4-hour hemodialysis session (post-dialysis) is 56 % higher when compared to subjects with normal renal function (see Table 18). The systemic exposure to rivaroxaban administered 2 hours prior to a 4-hour hemodialysis session with a dialysate flow rate of 600 mL/min and a blood flow rate in the range of 320 to 400 mL/min is 47 % higher compared to those with normal renal function. The extent of the increase is similar to the increase in patients with CrCl 15 to 50 mL/min taking rivaroxaban tablets 15 mg. Hemodialysis had no significant impact on rivaroxaban exposure. Protein binding was similar (86 % to 89 %) in healthy controls and ESRD subjects in this study.
Hepatic Impairment
The safety and pharmacokinetics of single-dose rivaroxaban tablets (10 mg) were evaluated in a study in healthy adult subjects (n=16) and adult subjects with varying degrees of hepatic impairment (see Figure 2). No patients with severe hepatic impairment (Child-Pugh C) were studied. Compared to healthy subjects with normal liver function, significant increases in rivaroxaban exposure were observed in subjects with moderate hepatic impairment (Child-Pugh B) (see Figure 2). Increases in pharmacodynamic effects were also observed [see Use in Specific Populations (8.7)].
No clinical data are available in pediatric patients with hepatic impairment.
Drug Interactions
In vitro studies indicate that rivaroxaban neither inhibits the major cytochrome P450 enzymes CYP1A2, 2C8, 2C9, 2C19, 2D6, 2J2, and 3A nor induces CYP1A2, 2B6, 2C19, or 3A. In vitro data also indicates a low rivaroxaban inhibitory potential for P-gp and ABCG2 transporters.
The effects of coadministered drugs on the pharmacokinetics of rivaroxaban exposure are summarized in Figure 3 [see Drug Interactions (7)].
Figure 3: Effect of Coadministered Drugs on the Pharmacokinetics of Rivaroxaban in Adults
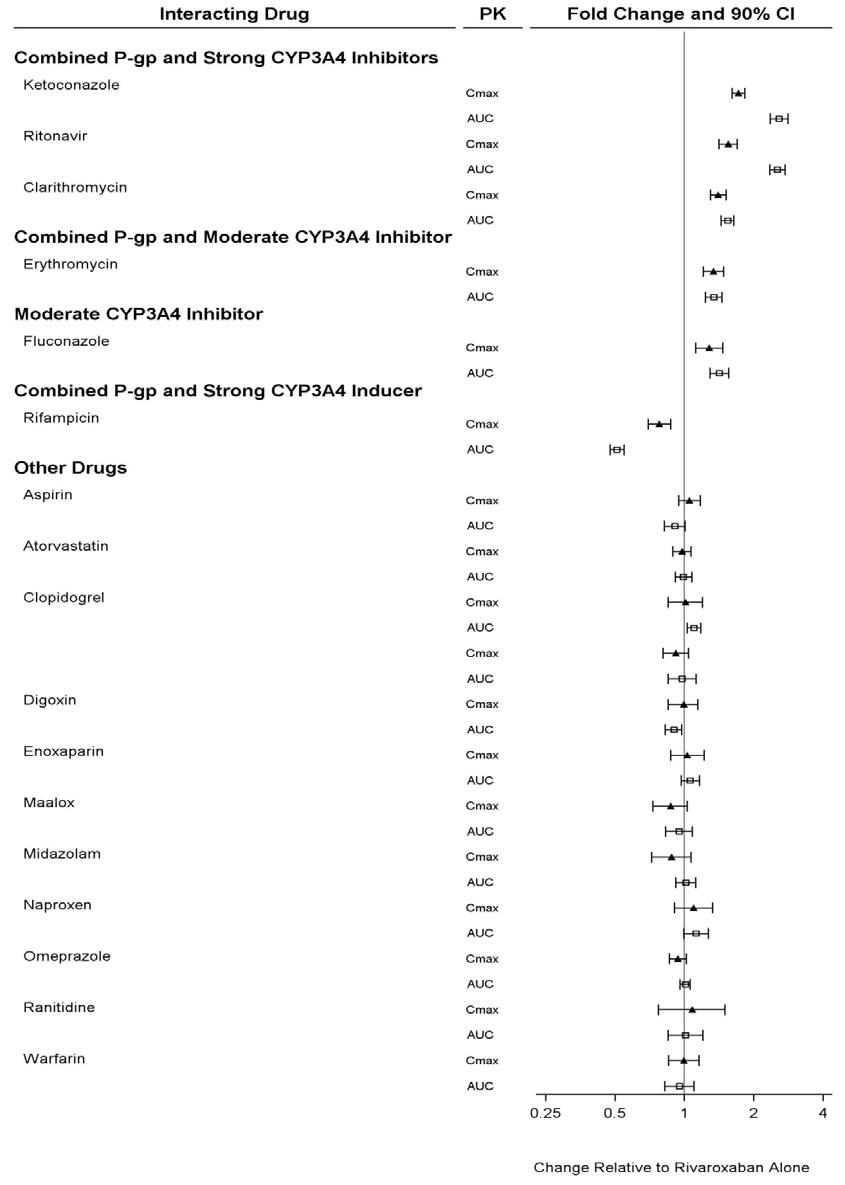
In a drug interaction study, single doses of enoxaparin (40 mg subcutaneous) and rivaroxaban tablets (10 mg) given concomitantly resulted in an additive effect on anti-factor Xa activity. In another study, single doses of warfarin (15 mg) and rivaroxaban tablets (5 mg) resulted in an additive effect on factor Xa inhibition and PT. Neither enoxaparin nor warfarin affected the pharmacokinetics of rivaroxaban (see Figure 3).
NSAIDs/Aspirin:
In ROCKET AF, concomitant aspirin use (almost exclusively at a dose of 100 mg or less) during the double-blind phase was identified as an independent risk factor for major bleeding. NSAIDs are known to increase bleeding, and bleeding risk may be increased when NSAIDs are used concomitantly with rivaroxaban tablets. Neither naproxen nor aspirin affected the pharmacokinetics of rivaroxaban (see Figure 3).
Clopidogrel:
In two drug interaction studies where clopidogrel (300 mg loading dose followed by 75 mg daily maintenance dose) and rivaroxaban tablets (15 mg single dose) were coadministered in healthy subjects, an increase in bleeding time to 45 minutes was observed in approximately 45 % and 30 % of subjects in these studies, respectively. The change in bleeding time was approximately twice the maximum increase seen with either drug alone. There was no change in the pharmacokinetics of either drug.
Drug-Disease Interactions with Drugs that Inhibit Cytochrome P450 3A Enzymes and Drug Transport Systems:
In a pharmacokinetic trial, rivaroxaban tablets were administered as a single dose in subjects with mild (CrCl = 50 to 79 mL/min) or moderate renal impairment (CrCl = 30 to 49 mL/min) receiving multiple doses of erythromycin (a combined P-gp and moderate CYP3A inhibitor). Compared to rivaroxaban tablets administered alone in subjects with normal renal function (CrCl >80 mL/min), subjects with mild and moderate renal impairment concomitantly receiving erythromycin reported a 76 % and 99 % increase in AUCinf and a 56 % and 64 % increase in Cmax, respectively. Similar trends in pharmacodynamic effects were also observed.
-
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Rivaroxaban was not carcinogenic when administered by oral gavage to mice or rats for up to 2 years. The systemic exposures (AUCs) of unbound rivaroxaban in male and female mice at the highest dose tested (60 mg/kg/day) were 1-and 2-times, respectively, the human exposure of unbound drug at the human dose of 20 mg/day. Systemic exposures of unbound drug in male and female rats at the highest dose tested (60 mg/kg/day) were 2-and 4-times, respectively, the human exposure.
Rivaroxaban was not mutagenic in bacteria (Ames-Test) or clastogenic in V79 Chinese hamster lung cells in vitro or in the mouse micronucleus test in vivo.
No impairment of fertility was observed in male or female rats when given up to 200 mg/kg/day of rivaroxaban orally. This dose resulted in exposure levels, based on the unbound AUC, at least 13 times the exposure in humans given 20 mg rivaroxaban daily.
-
14 CLINICAL STUDIES
14.6 Reduction of Risk of Major Cardiovascular Events in Patients with CAD
The evidence for the efficacy and safety of rivaroxaban tablets for the reduction in the risk of stroke, myocardial infarction, or cardiovascular death in patients with coronary artery disease (CAD) or peripheral artery disease (PAD) was derived from the double-blind, placebo-controlled Cardiovascular OutcoMes for People using Anticoagulation StrategieS trial (COMPASS) [NCT10776424]. A total of 27,395 patients were evenly randomized to rivaroxaban 2.5 mg orally twice daily plus aspirin 100 mg once daily, rivaroxaban 5 mg orally twice daily alone, or aspirin 100 mg once daily alone. Because the 5 mg dose alone was not superior to aspirin alone, only the data concerning the 2.5 mg dose plus aspirin are discussed below.
Patients with established CAD or PAD were eligible. Patients with CAD who were younger than 65 years of age were also required to have documentation of atherosclerosis involving at least two vascular beds or to have at least two additional cardiovascular risk factors (current smoking, diabetes mellitus, an estimated glomerular filtration rate [eGFR] <60 mL per minute, heart failure, or non-lacunar ischemic stroke ≥1 month earlier). Patients with PAD were either symptomatic with ankle brachial index <0.90 or had asymptomatic carotid artery stenosis ≥50 %, a previous carotid revascularization procedure, or established ischemic disease of one or both lower extremities. Patients were excluded for use of dual antiplatelet, other non-aspirin antiplatelet, or oral anticoagulant therapies, ischemic, non-lacunar stroke within 1 month, hemorrhagic or lacunar stroke at any time, or eGFR <15 mL/min.
The mean age was 68 years and 21 % of the subject population were ≥75 years. Of the included patients, 91 % had CAD (and will be referred to as the COMPASS CAD population), 27 % had PAD (and will be referred to as the COMPASS PAD population), and 18 % had both CAD and PAD. Of the patients with CAD, 69 % had prior MI, 60 % had prior percutaneous transluminal coronary angioplasty (PTCA)/atherectomy/ percutaneous coronary intervention (PCI), and 26 % had history of coronary artery bypass grafting (CABG) prior to study. Of the patients with PAD, 49 % had intermittent claudication, 27 % had peripheral artery bypass surgery or peripheral percutaneous transluminal angioplasty, 26 % had asymptomatic carotid artery stenosis > 50 %, and 4 % had limb or foot amputation for arterial vascular disease.
The mean duration of follow-up was 23 months. Relative to placebo, rivaroxaban tablets reduced the rate of the primary composite outcome of stroke, myocardial infarction or cardiovascular death: HR 0.76 (95 % CI: 0.66, 0.86; p=0.00004). In the COMPASS CAD population, the benefit was observed early with a constant treatment effect over the entire treatment period (see Table 26 and Figure 10).
A benefit-risk analysis of the data from COMPASS was performed by comparing the number of CV events (CV deaths, myocardial infarctions and non-hemorrhagic strokes) prevented to the number of fatal or life-threatening bleeding events (fatal bleeds + symptomatic non-fatal bleeds into a critical organ) in the rivaroxaban tablets group versus the placebo group. Compared to placebo, during 10,000 patient-years of treatment, rivaroxaban tablets would be expected to result in 70 fewer CV events and 12 additional life-threatening bleeds, indicating a favorable balance of benefits and risks.
The results in the COMPASS CAD population were consistent across major subgroups (see Figure 9).
Figure 9: Risk of Primary Efficacy Outcome by Baseline Characteristics in the COMPASS CAD Population (Intent-to-Treat Population)*
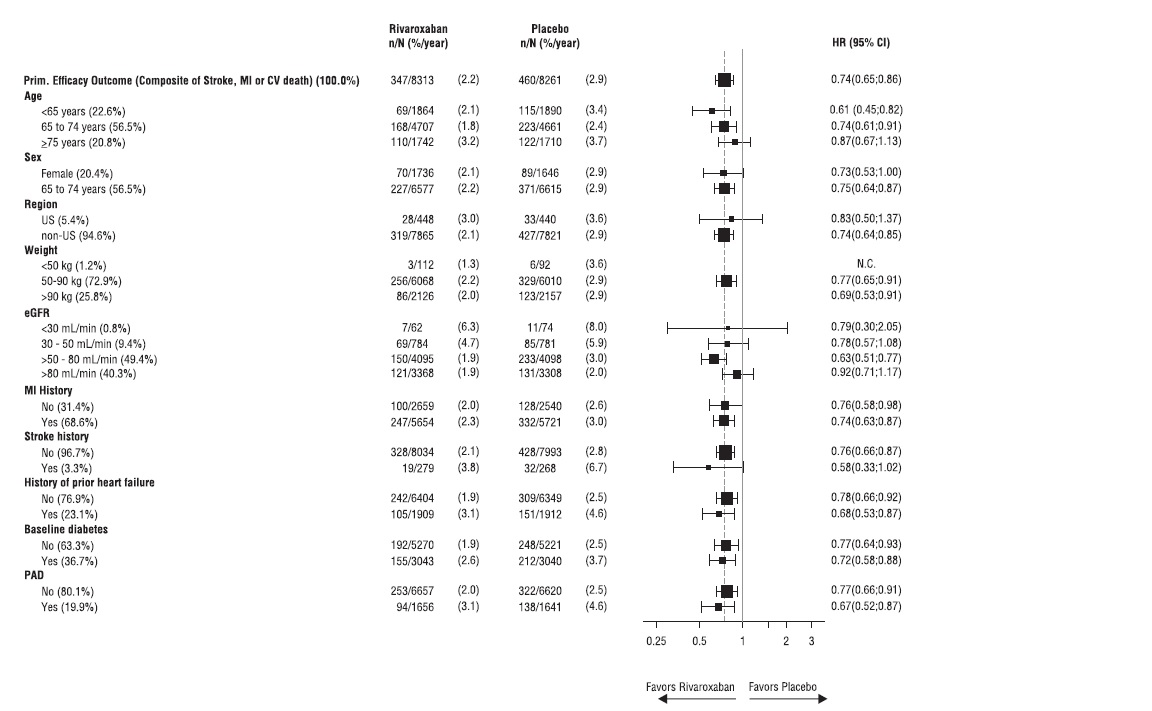
*All patients received aspirin 100 mg once daily as background therapy
Table 26: Efficacy results from COMPASS CAD Population * * Intention to treat analysis set, primary analyses.
† Treatment schedule: rivaroxaban tablets 2.5 mg twice daily vs placebo. All patients received aspirin 100 mg once daily as background therapy.
‡ Rivaroxaban tablets vs. placebo.
§ Coronary heart disease death: death due to acute MI, sudden cardiac death, or CV procedure.
¶ CV death includes CHD death, or death due to other CV causes or unknown death.
# Acute limb ischemia is defined as limb-threatening ischemia leading to an acute vascular intervention (i.e., pharmacologic, peripheral arterial surgery/reconstruction, peripheral angioplasty/stent, or amputation).
CHD: coronary heart disease, CI: confidence interval; CV: cardiovascular; MI: myocardial infarction
Event
Rivaroxaban Tablets† N=8313
Placebo † N=8261
Hazard Ratio (95 % CI) ‡
n (%)
Event Rate (%/year)
n (%)
Event Rate (%/year)
Stroke, MI or CV death
347 (4.2)
2.2
460 (5.6)
2.9
0.74 (0.65, 0.86)
- Stroke
74 (0.9)
0.5
130 (1.6)
0.8
0.56 (0.42, 0.75)
- MI
169 (2)
1.1
195 (2.4)
1.2
0.86 (0.70, 1.05)
- CV death
139 (1.7)
0.9
184 (2.2)
1.1
0.75 (0.60, 0.93)
Coronary heart disease death, MI, ischemic stroke, acute limb ischemia
299 (3.6)
1.9
411 (5)
2.6
0.72 (0.62, 0.83)
- Coronary heart disease death§
80 (1)
0.5
107 (1.3)
0.7
0.74 (0.55, 0.99)
- Ischemic stroke
56 (0.7)
0.3
114 (1.4)
0.7
0.49 (0.35, 0.67)
- Acute limb ischemia#
13 (0.2)
0.1
27 (0.3)
0.2
0.48 (0.25, 0.93)
CV death¶, MI, ischemic stroke, acute limb ischemia
349 (4.2)
2.2
470 (5.7)
3
0.73 (0.64, 0.84)
All-cause mortality
262 (3.2)
1.6
339 (4.1)
2.1
0.77 (0.65, 0.90)
Figure 10: Time to First Occurrence of Primary Efficacy Outcome (Stroke, Myocardial Infarction, Cardiovascular Death) in the COMPASS CAD Population*
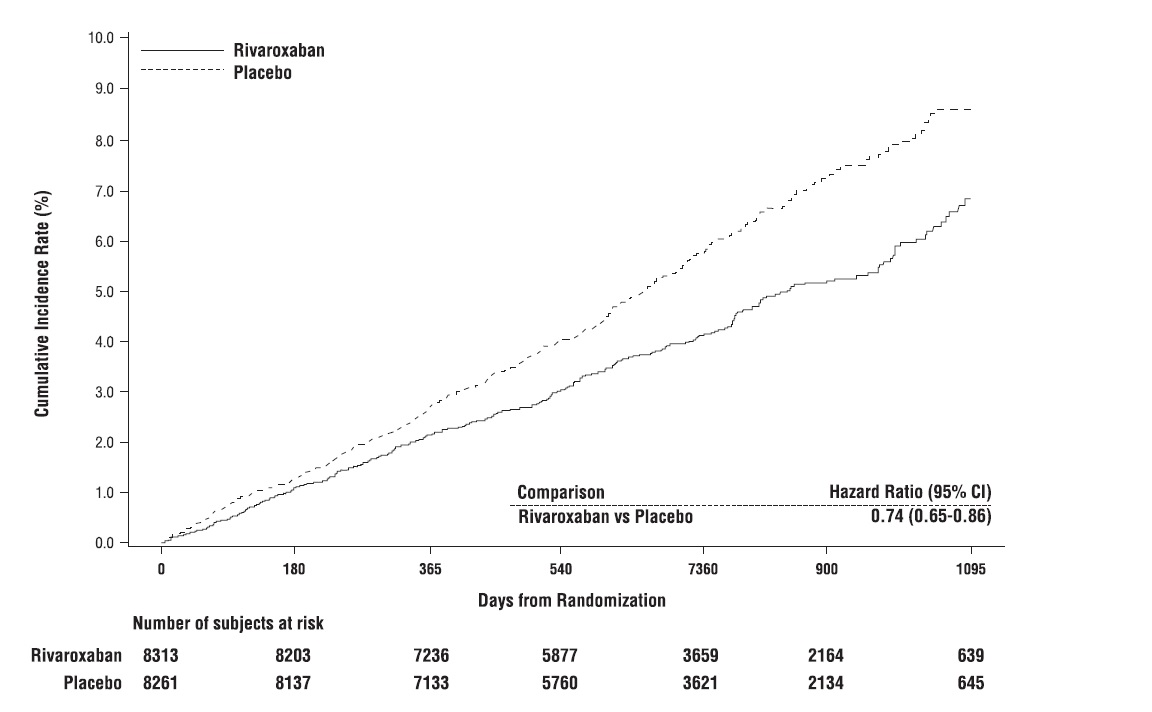
*All patients received aspirin 100 mg once daily as background therapy.
CI: confidence interval
14.7 Reduction of Risk of Major Thrombotic Vascular Events in Patients with PAD, Including Patients after Lower Extremity Revascularization due to Symptomatic PAD
The efficacy and safety of rivaroxaban tablets 2.5 mg orally twice daily versus placebo on a background of aspirin 100 mg once daily in patients with PAD were evaluated in the COMPASS study (n=4996) and will be referred to as the COMPASS PAD population [see Clinical Studies (14.6)].
The efficacy and safety of rivaroxaban tablets were also evaluated for the reduction in the risk of the composite endpoint of myocardial infarction, ischemic stroke, cardiovascular death, acute limb ischemia (ALI), and major amputation of a vascular etiology in patients undergoing a lower extremity infrainguinal revascularization procedure due to symptomatic peripheral artery disease (PAD) in the double-blinded, placebo-controlled Vascular Outcomes studY of ASA alonG with rivaroxaban in Endovascular or surgical limb Revascularization for peripheral artery disease (PAD) trial (VOYAGER) [NCT02504216]. A total of 6,564 patients were equally randomized to rivaroxaban tablets 2.5 mg orally twice daily vs placebo on a background therapy of aspirin 100 mg once daily.
Eligible patients included adults who were at least 50 years of age with documented moderate to severe symptomatic lower extremity atherosclerotic PAD who had a successful peripheral surgical procedure and/or endovascular procedure with or without clopidogrel (up to a maximum of 6 months was allowed; median duration of therapy was 31 days). Patients had either a prior history of limb revascularization with ankle brachial index ≤0.85 or no prior history of limb revascularization with ankle brachial index ≤0.80. Patients in need of dual antiplatelet for >6 months, or any additional antiplatelet other than aspirin and clopidogrel, or oral anticoagulant, as well as patients with a history of intracranial hemorrhage, stroke, or transient ischemic attack (TIA), or patients with eGFR <15 mL/min were excluded.
The mean age was 67 years and 20 % of the subject population was ≥75 years. Of the included patients, 35 % had surgical revascularization, 47 % had endovascular revascularization with clopidogrel, and 18 % endovascular revascularization without clopidogrel. The median duration of follow-up was 30.8 months.
Rivaroxaban tablets 2.5 mg twice daily were superior to placebo in reducing the rate of the primary composite outcome of myocardial infarction, ischemic stroke, cardiovascular death, acute limb ischemia (ALI), and major amputation of a vascular etiology. The primary efficacy outcome and its components are provided in Table 27. The Kaplan-Meier plot for the primary efficacy outcome can be seen in Figure 11. The secondary efficacy outcomes were tested for superiority in a prespecified, hierarchical order and the first five of seven endpoints were significantly reduced in the rivaroxaban treatment arm (see Table 27). Compared to placebo during 10,000 patient-years of treatment, rivaroxaban tablets would be expected to result in 181 fewer primary outcome events and 29 more TIMI major bleeding events, indicating a favorable balance of benefits and risks.
Figure 11: Time to First Occurrence of Primary Efficacy Outcome (Myocardial Infarction, Ischemic Stroke, Cardiovascular Death, Acute Limb Ischemia, Major Amputation due to Vascular Origins) in VOYAGER*
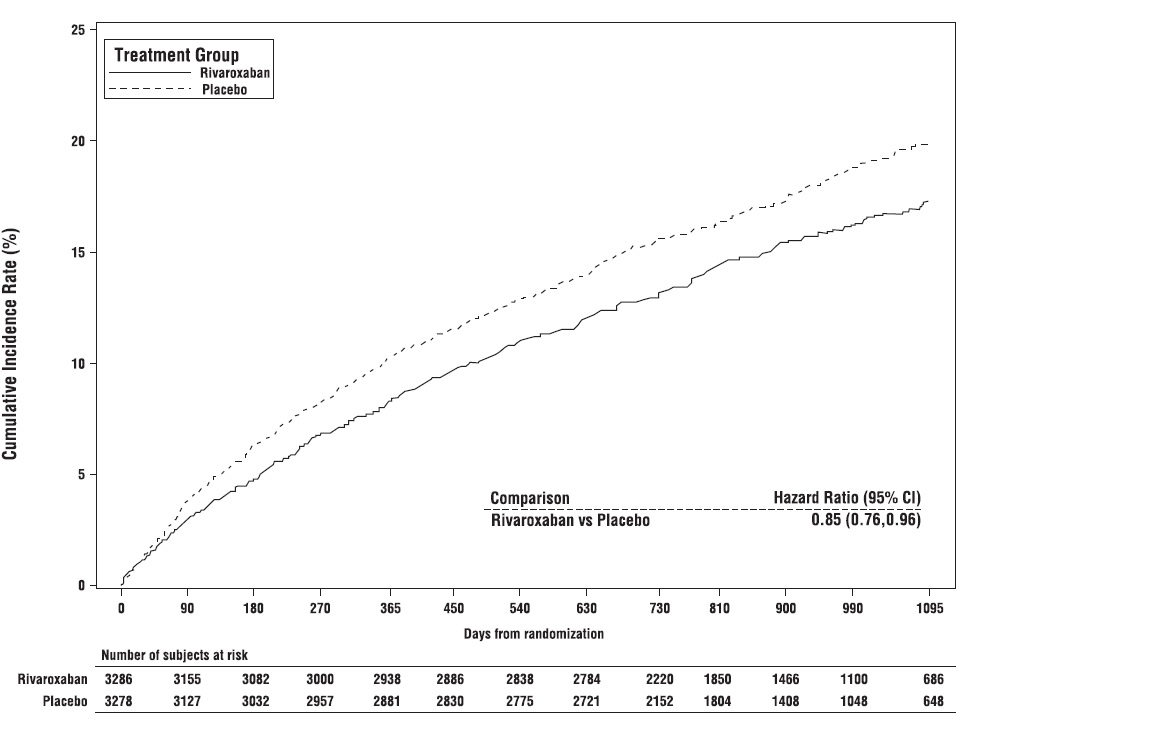
*All patients received aspirin 100 mg once daily as background therapy.
Figure 12 shows the risk of primary efficacy outcome across major subgroups. Subgroup analyses must be interpreted cautiously, as differences can reflect the play of chance among a large number of analyses. The primary efficacy endpoint generally shows homogeneous results across subgroups.
Figure 12: Risk of Primary Efficacy Outcome by Baseline Characteristics in VOYAGER (Intent-to-Treat Population)*
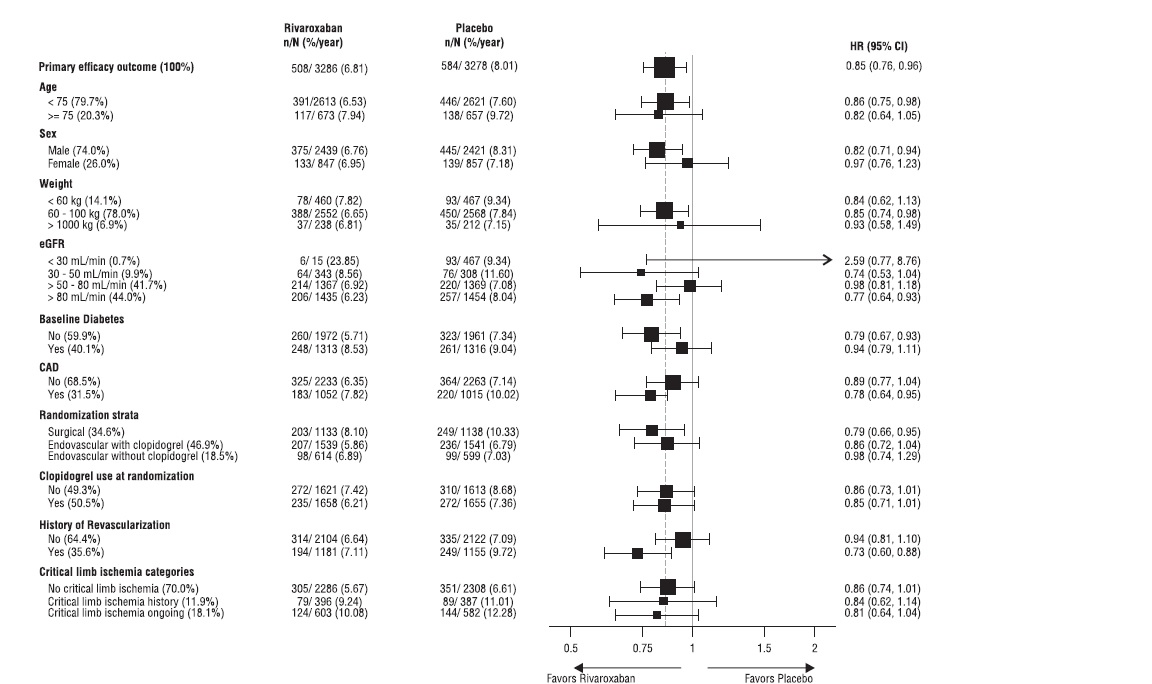
*All patients received aspirin 100 mg once daily as background therapy
Table 27 provides the efficacy event rates for the prespecified endpoints in VOYAGER and similar endpoints in the COMPASS PAD population.
Table 27: Efficacy Results in VOYAGER (Intent-to-Treat Population) and COMPASS PAD Efficacy endpoints in COMPASS PAD were analysed according to the pre-specified endpoints in VOYAGER when applicable.
* Rivaroxaban Tablets vs. placebo.
† Two-sided p-values
‡ Major thrombotic vascular event is the composite of MI, ischemic stroke, CV death, ALI, and major amputation of a vascular etiology.
§ Ischemic stroke for VOYAGER included stroke of uncertain/unknown etiology whereas COMPASS only included ischemic stroke.
¶ CV death includes Coronary Heart Disease death, or death due to other CV causes or sudden cardiac arrest and unknown death.
# Adjudicated events in VOYAGER and investigator reported events in COMPASS
Þ Secondary outcomes for VOYAGER were tested sequentially.
ß CHD death includes death due to sudden cardiac death, MI, or coronary revascularization procedure
à Unplanned index limb revascularization for recurrent limb ischemia was not captured in COMPASS study.
è Investigator reported in VOYAGER and adjudicated events in COMPASS
ALI=acute limb ischemia, CHD=coronary heart disease; CI=confidence interval, CV=cardiovascular; MI=myocardial infarction, VTE=venous thromboembolism
VOYAGER
COMPASS PAD
Rivaroxaban Tablets N=3286
Placebo N=3278
Hazard Ratio (95 % CI)*p-value†
Rivaroxaban Tablets N=2492
Placebo N=2504
Hazard Ratio (95 % CI)*
Outcome Components
Event Rate (%/year)
Event Rate (%/year)
5-Component Outcome (Major thrombotic vascular events)‡
6.8
8
0.85 (0.76, 0.96) p=0.0085
3.4
4.8
0.71 (0.57, 0.87)
MI
1.7
1.9
0.88 (0.7, 1.12)
1.1
1.5
0.76 (0.53, 1.09)
Ischemic Stroke§
0.9
1
0.87 (0.63, 1.19)
0.5
0.9
0.55 (0.33, 0.93)
CV death¶
2.5
2.2
1.14 (0.93, 1.4)
1.4
1.7
0.82 (0.59, 1.14)
ALI
2
3
0.67 (0.55, 0.82)
0.4
0.8
0.56 (0.32, 0.99)
Major amputation of a vascular etiology#
1.3
1.5
0.89 (0.68, 1.16)
0.2
0.6
0.4 (0.2, 0.79)
VOYAGER Secondary Efficacy OutcomesÞ
MI, ischemic stroke, CHD death,ß ALI, and major amputation due to vascular etiology
5.8
7.3
0.8 (0.71, 0.91) p=0.0008
2.8
4.2
0.66 (0.53, 0.83)
Unplanned index limb revascularization for recurrent limb ischemiaà
8.4
9.5
0.88 (0.79, 0.99) p=0.028
N/A
N/A
N/A
Hospitalization for a coronary or peripheral cause of a thrombotic nature#
3.5
4.8
0.72 (0.62, 0.85) p<0.0001
1.7
2.9
0.58 (0.44, 0.77)
MI, ischemic stroke, all-cause mortality, ALI, and major amputation due to vascular etiology
8.2
9.3
0.89 (0.79, 0.99) p=0.029
4.8
6
0.8 (0.67, 0.96)
MI, all-cause stroke, CV death, ALI, and major amputation due to vascular etiology
6.9
8.1
0.86 (0.76, 0.96) p=0.01
3.4
4.9
0.7 (0.57, 0.86)
All-cause mortality
4
3.7
1.08 (0.92, 1.27)
2.8
3.1
0.91 (0.72, 1.16)
VTE eventsè
0.3
0.5
0.61 (0.37, 1)
0.2
0.3
0.67 (0.3, 1.49)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Rivaroxaban Tablets USP, 2.5 mg available in the packages listed below:
- 2.5 mg tablets: Light yellow colored, round shaped, biconvex, film-coated tablets, debossed with "C4" on one side and "L" on the other side. The tablets are supplied in the packages listed:
NDC: 68180-709-06 Bottle containing 30 tablets
NDC: 68180-709-09 Bottle containing 90 tablets
Store at 25°C (77°F) or room temperature; excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Keep out of the reach of children.
-
17 PATIENT COUNSELING INFORMATION
For the tablets, advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide).
Instructions for Patient Use
- Advise patients to take rivaroxaban tablets only as directed.
- Remind patients to not discontinue rivaroxaban tablets without first talking to their healthcare professional.
- Advise patients who cannot swallow the tablets whole to crush rivaroxaban tablets and combine with a small amount of applesauce followed by food [see Dosage and Administration (2.6)].
- For patients requiring an NG tube or gastric feeding tube, instruct the patient or caregiver to crush the rivaroxaban tablets and mix it with a small amount of water before administering via the tube [see Dosage and Administration (2.6)].
- If a dose is missed, advise the patient according to the instructions in the Full Prescribing Information based on their dosing schedule [see Dosage and Administration (2.5)].
- Advise patients to report any unusual bleeding or bruising to their physician. Inform patients that it might take them longer than usual to stop bleeding, and that they may bruise and/or bleed more easily when they are treated with rivaroxaban tablets [see Warnings and Precautions (5.2)] .
- If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs or platelet inhibitors, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately [see Boxed Warning] .
Invasive or Surgical Procedures
Instruct patients to inform their healthcare professional that they are taking rivaroxaban tablets before any invasive procedure (including dental procedures) is scheduled.
Concomitant Medication and Herbals
Advise patients to inform their physicians and dentists if they are taking, or plan to take, any prescription or over-the-counter drugs or herbals, so their healthcare professionals can evaluate potential interactions [see Drug Interactions (7)].
Pregnancy and Pregnancy-Related Hemorrhage
- Advise patients to inform their physician immediately if they become pregnant or intend to become pregnant during treatment with rivaroxaban tablets [see Use in Specific Populations (8.1)].
- Advise pregnant women receiving rivaroxaban tablets to immediately report to their physician any bleeding or symptoms of blood loss [see Warnings and Precautions (5.7)].
Advise patients to discuss with their physician the benefits and risks of rivaroxaban tablets for the mother and for the child if they are nursing or intend to nurse during anticoagulant treatment [see Use in Specific Populations (8.2)].
Females and Males of Reproductive Potential
Advise patients who can become pregnant to discuss pregnancy planning with their physician [see Use in Specific Populations (8.3)].
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
MADE IN INDIA
July 2025
Dispense with Medication Guide available at: www.lupin.com/rivaroxabantab-mg.pdf
-
MEDICATION GUIDE
MEDICATION GUIDE
Rivaroxaban (RIV-a-ROX-a-ban) Tablets, USP
What is the most important information I should know about rivaroxaban tablets ?
Rivaroxaban tablets may cause serious side effects, including:
- Increased risk of blood clots if you stop taking rivaroxaban tablets.People with atrial fibrillation (a type of irregular heart beat) that is not caused by a heart valve problem (non-valvular) are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. Rivaroxaban tablets lower your chance of having a stroke by helping to prevent clots from forming. If you stop taking rivaroxaban tablets, you may have increased risk of forming a clot in your blood.
Do not stop taking rivaroxaban tablets without talking to the doctor who prescribes it for you. Stopping rivaroxaban tablets increases your risk of having a stroke. If you have to stop taking rivaroxaban tablets, your doctor may prescribe another blood thinner medicine to prevent a blood clot from forming.
- Increasedrisk of bleeding.Rivaroxaban tablets can cause bleeding which can be serious and may lead to death. This is because rivaroxaban is a blood thinner medicine (anticoagulant) that lowers blood clotting. During treatment with rivaroxaban tablets you are likely to bruise more easily, and it may take longer for bleeding to stop. You may have a higher risk of bleeding if you take rivaroxaban tablets and have certain other medical problems.
You may have a higher risk of bleeding if you take rivaroxaban tablets and take other medicines that increase your risk of bleeding, including:
- aspirin or aspirin containing products
- long-term (chronic) use of non-steroidal anti-inflammatory drugs (NSAIDs)
- warfarin sodium (Coumadin®, Jantoven®)
- any medicine that contains heparin
- clopidogrel (Plavix®)
- selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs)
- other medicines to prevent or treat blood clots
Tell your doctor if you take any of these medicines. Ask your doctor or pharmacist if you are not sure if your medicine is one listed above.
Call your doctor or get medical help right away if you or your child develop any of these signs or symptoms of bleeding:
ο unexpected bleeding or bleeding that lasts a long time, such as:
■ nose bleeds that happen often
■ unusual bleeding from the gums
■ menstrual bleeding that is heavier than normal or vaginal bleeding
ο bleeding that is severe or you cannot control
ο red, pink or brown urine
ο bright red or black stools (looks like tar)
ο cough up blood or blood clots
ο vomit blood or your vomit looks like "coffee grounds"
ο headaches, feeling dizzy or weak
ο pain, swelling, or new drainage at wound sites
ο left upper belly (abdominal) pain, pain below the left rib cage or at the tip of your left shoulder or diffuse abdominal discomfort (these may be symptoms of rupture of the spleen)
- Spinal or epidural blood clots (hematoma).People who take a blood thinner medicine (anticoagulant) like rivaroxaban, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:
о a thin tube called an epidural catheter is placed in your back to give you certain medicine
о you take NSAIDs or a medicine to prevent blood from clotting
о you have a history of difficult or repeated epidural or spinal punctures
о you have a history of problems with your spine or have had surgery on your spine
If you take rivaroxaban tablets and receive spinal anesthesia or have a spinal puncture, your doctor should watch you closely for symptoms of spinal or epidural blood clots.
Tell your doctor right away if you have
back pain
tingling
numbness
muscle weakness (especially in your legs and feet)
loss of control of the bowels or bladder (incontinence).
Rivaroxaban tablets are not for use in people with artificial heart valves.
Rivaroxaban tablets are not for use in people with antiphospholipid syndrome (APS), especially with positive triple antibody testing.
Rivaroxaban tablets are used with low dose aspirin to:
- reduce the risk of serious heart problems, heart attack and stroke in adults with coronary artery disease (a condition where the blood supply to the heart is reduced or blocked)
- reduce the risk of a sudden decrease in blood flow to the legs, major amputation, serious heart problems or stroke in adults with peripheral artery disease (a condition where the blood flow to the legs is reduced) and includes adults who have recently had a procedure to improve blood flow to the legs.
Do not take rivaroxaban tablets if you or your child:
- currently have certain types of abnormal bleeding. Talk to your doctor before taking rivaroxaban tablets if you currently have unusual bleeding.
- are allergic to rivaroxaban or any of the ingredients in rivaroxaban tablets. See the end of this Medication Guide for a complete list of ingredients in rivaroxaban tablets.
Before taking rivaroxaban tablets, tell your doctor about all of your medical conditions, including if you or your child:
- have or ever had bleeding problems
- have liver or kidney problems
- have antiphospholipid syndrome (APS)
- are pregnant or plan to become pregnant. It is not known if rivaroxaban tablets will harm your unborn baby.
- Tell your doctor right away if you become pregnant during treatment with rivaroxaban tablets. Taking rivaroxaban tablets while you are pregnant may increase the risk of bleeding in you or in your unborn baby.
- Females who are able to become pregnant: Talk with your doctor about pregnancy planning during treatment with rivaroxaban tablets. Talk with your doctor about your risk for severe uterine bleeding if you are treated with blood thinner medicines, including rivaroxaban tablets.
- If you take rivaroxaban tablets during pregnancy tell your doctor right away if you have any signs or symptoms of bleeding or blood loss. See"What is the most important information I should know about rivaroxaban tablets?" for signs and symptoms of bleeding.
- are breastfeeding or plan to breastfeed. Rivaroxaban can pass into your breast milk. Talk to your doctor about the best way to feed your baby during treatment with rivaroxaban tablets.
Tell all of your doctors and dentists that you or your child are taking rivaroxaban tablets. They should talk to the doctor who prescribed rivaroxaban tablets for you before you have any surgery, medical or dental procedure.
Tell your doctor about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some of your other medicines may affect the way rivaroxaban tablets works causing side effects. Certain medicines may increase your risk of bleeding. See "What is the most important information I should know about rivaroxaban tablets?"
Especially tell your doctor if you take or your child:
- ketoconazole
- ritonavir
- erythromycin
- carbamazepine
- phenytoin
- rifampin
- St. John's wort
How should I take rivaroxaban tablets?
- Take rivaroxaban tablets exactly as prescribed by your doctor.
- Do not change your dose or stop taking rivaroxaban tablets unless your doctor tells you to. Your doctor may change your dose if needed.
- Your doctor will decide how long you should take rivaroxaban tablets.
- Rivaroxaban tablets may need to be stopped for one or more days before any surgery or medical or dental procedure. Your doctor will tell you when to stop taking rivaroxaban tablets and when to start taking rivaroxaban tablets again after your surgery or procedure.
- If you need to stop taking rivaroxaban tablets for any reason, talk to the doctor who prescribed rivaroxaban tablets to you to find out when you should stop taking it. Do not stop taking rivaroxaban tablets without first talking to the doctor who prescribes it to you.
- If you have difficulty swallowing rivaroxaban tablets tablets whole, talk to your doctor about other ways to take rivaroxaban tablets.
- Do not run out of rivaroxaban tablets. Refill your prescription of rivaroxaban tablets before you run out. When leaving the hospital following a hip or knee replacement, be sure that you will have rivaroxaban tablets available to avoid missing any doses.
- If you take too much rivaroxaban tablets, go to the nearest hospital emergency room or call your doctor right away.
If you take rivaroxaban tablets for:
- Reducing the risk of serious heart problems, heart attack and stroke in coronary artery disease:
■ Take rivaroxaban tablets, 2.5 mg 2 times a day with or without food.
■ If you miss a dose of rivaroxaban tablets, take your next dose at your regularly scheduled time.
■ Take aspirin 75 to 100 mg once daily as instructed by your doctor.
- Reducing the risk of a sudden decrease in blood flow to the legs, major amputation, serious heart problems or stroke in people with peripheral artery disease including those who have recently had a procedure to improve blood flow to the legs:
■ Take rivaroxaban tablets 2.5 mg 2 times a day with or without food.
■ If you miss a dose of rivaroxaban tablets, take your next dose at your regularly scheduled time.
■ Take aspirin 75 mg to 100 mg 1 time a day as instructed by your doctor.
What are the possible side effects of rivaroxaban tablets?
Rivaroxaban tablets may cause serious side effects:
- See "What is the most important information I should know about rivaroxaban tablets? "
The most common side effect of rivaroxaban tablets in adults was bleeding.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effect to Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or visit www.lupin.com.
How should I store rivaroxaban tablets?
Store rivaroxaban tablets at room temperature between 59°F to 86°F (15°C to 30°C).
Keep rivaroxaban tablets and all medicines out of the reach of children.
General information about the safe and effective use of rivaroxaban tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use rivaroxaban tablets for a condition for which it was not prescribed. Do not give rivaroxaban tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about rivaroxaban tablets that is written for health professionals.
What are the ingredients in rivaroxaban tablets?
Active ingredient: rivaroxaban
Inactive ingredients tablets: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.
The proprietary film coating mixture for rivaroxaban tablets, 2.5 mg is Opadry®Yellow containing hypromellose, iron oxide yellow, macrogol / PEG and titanium dioxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
® The brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
MADE IN INDIA
July 2025
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RIVAROXABAN
rivaroxaban tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68180-709 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIVAROXABAN (UNII: 9NDF7JZ4M3) (RIVAROXABAN - UNII:9NDF7JZ4M3) RIVAROXABAN 2.5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color YELLOW (light yellow) Score no score Shape ROUND Size 5mm Flavor Imprint Code C4;L Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68180-709-09 90 in 1 BOTTLE; Type 0: Not a Combination Product 03/06/2025 2 NDC: 68180-709-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/06/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208555 03/06/2025 Labeler - Lupin Pharmaceuticals, Inc. (089153071) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 862272739 MANUFACTURE(68180-709) , PACK(68180-709)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.