BANOBAGI BABY FACE INJECTIONMASK- glycerin liquid
Banobagi Baby Face InjectionMask by
Drug Labeling and Warnings
Banobagi Baby Face InjectionMask by is a Otc medication manufactured, distributed, or labeled by Banobagi Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Water, Methylpropanediol, Caprylic/Capric Triglyceride, Acetyl Hexapeptide-8, Copper Tripeptide-1, Leontopodium Alpinum Callus Culture Extract, Glycine, Serine, Glutamic acid, Aspartic acid, Leucine, Alanine, Lysine, Arginine, Tyrosine, Phenylalanine, Threonine, Proline, Valine, Isoleucine, Histidine, Methionine, Cysteine, Bixa Orellana Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Glycine Soja (Soybean) Oil, Tocopherol, Dimethicone, Polysorbate 60, Xanthan Gum, Sorbitan Sesquioleate, Allantoin, Panthenol, Polyacrylate-13, Polyisobutene, Polysorbate 20, Carbomer, Potassium Hydroxide, 1,2-Hexanediol, Hydroxyacetophenone, Disodium EDTA, Fragrance
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
If abnormal symptoms or side effects such as red spots, swelling, or itching are present in the cosmetics or in the direct sunlight after use, consult a specialist
Do not use in wounded areas
Precautions for storage and handlingKeep out of reach of children
Keep away from direct sunlightAvoid the use of eye area
- DOSAGE & ADMINISTRATION
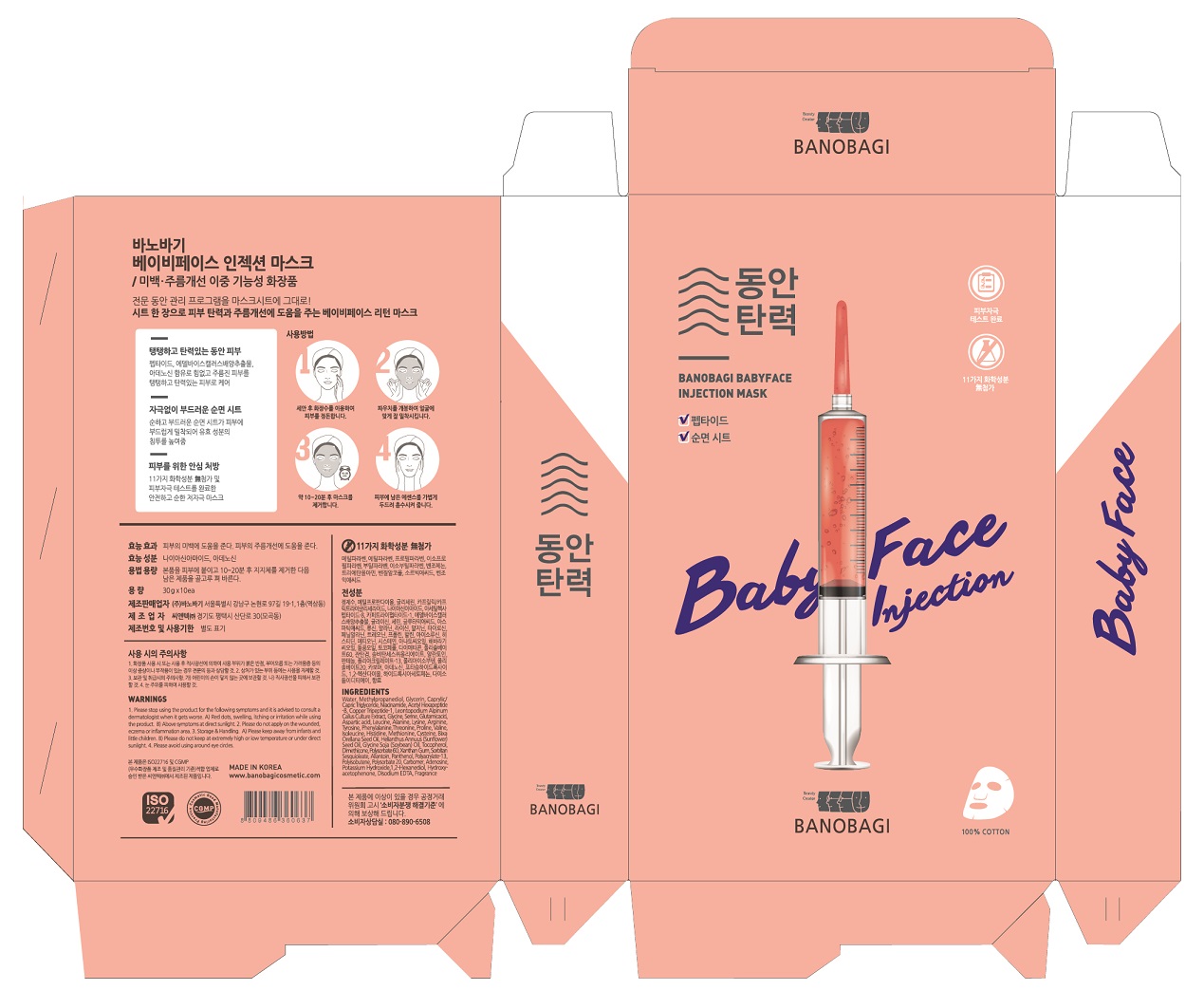
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BANOBAGI BABY FACE INJECTIONMASK
glycerin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72001-0006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALLANTOIN (UNII: 344S277G0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72001-0006-1 30 g in 1 POUCH; Type 0: Not a Combination Product 06/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part347 06/01/2018 Labeler - Banobagi Co., Ltd. (694436539) Registrant - Banobagi Co., Ltd. (694436539) Establishment Name Address ID/FEI Business Operations Cntec inc 688305034 manufacture(72001-0006) Establishment Name Address ID/FEI Business Operations Banobagi Co., Ltd. 694436539 label(72001-0006)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.