LIDOCAINE- lidocaine 4% cream
Lidocaine by
Drug Labeling and Warnings
Lidocaine by is a Otc medication manufactured, distributed, or labeled by AvKARE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient:
- Purpose
- Uses
-
WARNINGS
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
- Directions
- Other Information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
NDC: 42291-399-30
Lidocaine
4%
Cream
Topical Anesthetic CreamNet Wt. 30 gm
Drug Facts
Active ingredient Purpose
Lidocaine 4% w/w
............................Topical anesthetic
Uses
Temporarily relieves pain and
itching due to:
❚ minor cuts ❚ minor scrapes
❚ sunburn ❚ minor burns
❚ minor skin irritations
❚ insect bites
Warnings
For external use only.
When using this product
❚ do not use in or near the eyes
❚ do not use in large quantities,
particularly over raw surfaces
or blistered ares
Stop use and ask a doctor if
❚ allergic reaction occurs
❚ condition worsens or does not
improve within 7 days
❚ symptoms clear up and return
within a few days
❚ redness, irritation, swelling, pain
or other symptoms begin or
increase
Keep out of reach of children.
If swallowed, get medical help or
contact a Poison Control Center
right away
Directions
❚ Adults and children 2 years
and older: Apply externally to
the affected area up to 3-4 times
daily.
❚ Children under 2 years of age:
Consult a physician
Other Information
❚ Store at USP controlled room
temperature 20°-25°C
(68° - 77°F)
Inactive Ingredients
Ceteareth-6, ceteareth-25, cetyl
alcohol, methylparaben, paraffin,
propylene glycol, propylparaben,
purified water, stearyl alcohol,
xanthan gum.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AV 03/18 Rev: 03/18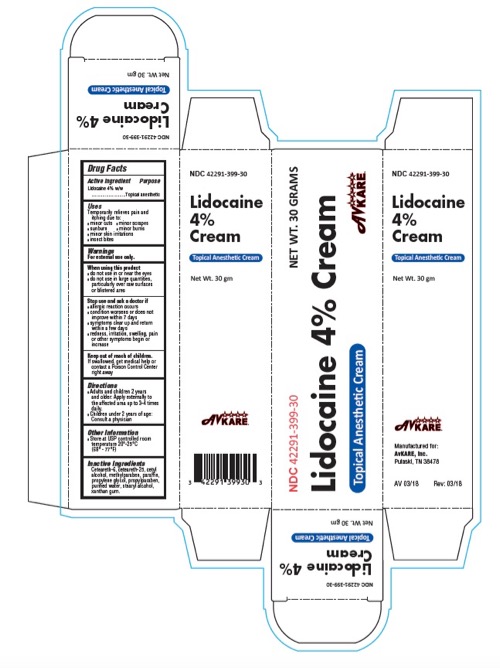

-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine 4% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42291-399 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength PARAFFIN (UNII: I9O0E3H2ZE) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) CETEARETH-25 (UNII: 8FA93U5T67) PROPYLPARABEN (UNII: Z8IX2SC1OH) CETEARETH-6 (UNII: 2RJS3559D3) CETYL ALCOHOL (UNII: 936JST6JCN) METHYLPARABEN (UNII: A2I8C7HI9T) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42291-399-30 1 in 1 CARTON 07/10/2018 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/10/2018 Labeler - AvKARE, Inc. (796560394)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.