2000DL-CL30- promo spf30 lotion
2000DL-CL30 by
Drug Labeling and Warnings
2000DL-CL30 by is a Otc medication manufactured, distributed, or labeled by Tropical Enterprises International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
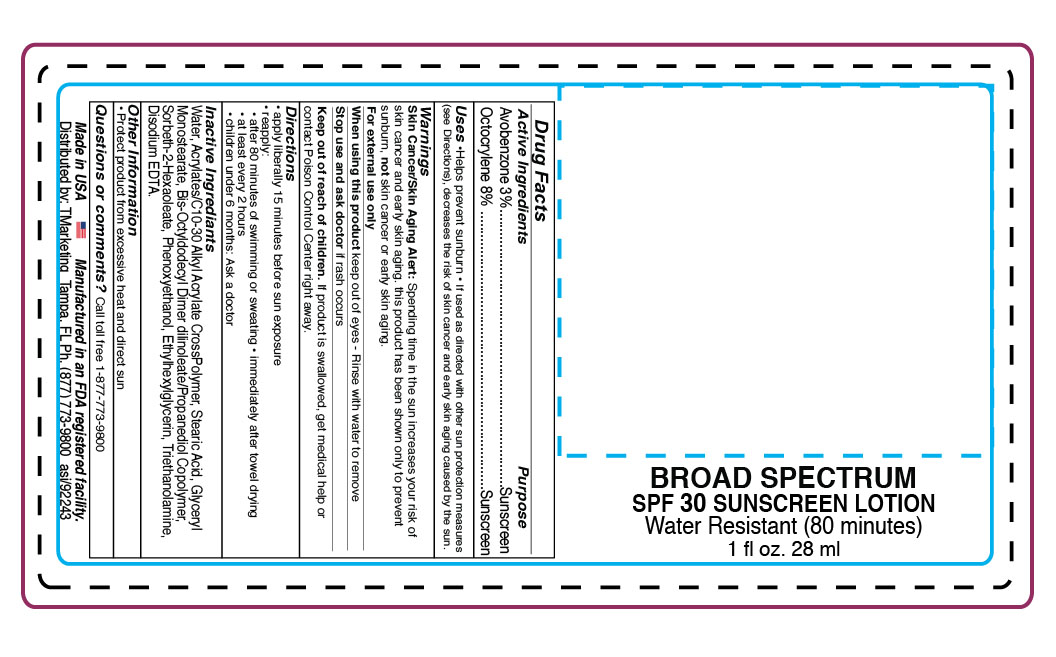
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
-
WARNINGS
WARNINGS: Skin Cancer/ Sking Aging Alert: Spending time in the sun
increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer and early skin aging.
For external use only. Do not use on damaged or broken skin.
When using this product avoid contact with eyes. If contact with eyes occurs, flush with water to remove.
Stop use and ask a doctor if irritation or rash develops or persists.
Keep out of reach of children. If product is swallowed, seek immediate medical attention or contact a Poison Control Center right away.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
2000DL-CL30
promo spf30 lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58418-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 8 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 17-HYDROXYSTEARIC ACID (UNII: O0217BD3D2) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SORBETH-12 HEXAOLEATE (UNII: F3ECF77W74) BIS-OCTYLDODECYL DIMER DILINOLEATE/PROPANEDIOL COPOLYMER (UNII: TY3J98ZR7R) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) PROPYLPARABEN (UNII: Z8IX2SC1OH) DILINOLEIC ACID/PROPANEDIOL COPOLYMER (UNII: VI8BUP90UI) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) GLATIRAMER ACETATE (UNII: 5M691HL4BO) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58418-002-10 10 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 10/01/2018 2 NDC: 58418-002-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 3 NDC: 58418-002-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 4 NDC: 58418-002-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 5 NDC: 58418-002-08 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 6 NDC: 58418-002-80 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2018 7 NDC: 58418-002-12 360 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2018 8 NDC: 58418-002-16 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2018 9 NDC: 58418-002-64 1920 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2018 10 NDC: 58418-002-28 3840 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2018 11 NDC: 58418-002-00 1.5 mL in 1 PACKET; Type 0: Not a Combination Product 02/02/2020 12 NDC: 58418-002-70 211200 mL in 1 DRUM; Type 0: Not a Combination Product 02/02/2020 13 NDC: 58418-002-32 40960 mL in 1 CONTAINER, FLEXIBLE INTERMEDIATE BULK; Type 0: Not a Combination Product 02/02/2020 14 NDC: 58418-002-15 45 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/02/2020 15 NDC: 58418-002-18 52 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/02/2020 16 NDC: 58418-002-55 30 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/01/2018 Labeler - Tropical Enterprises International, Inc. (091986179) Registrant - Tropical Enterprises International, Inc. (091986179) Establishment Name Address ID/FEI Business Operations Tropical Enterprises International, Inc. 091986179 relabel(58418-002) , repack(58418-002) , pack(58418-002) , manufacture(58418-002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.