RestoreZ by Sage Products LLC RestoreZ
RestoreZ by
Drug Labeling and Warnings
RestoreZ by is a Otc medication manufactured, distributed, or labeled by Sage Products LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RESTOREZ- zinc oxide cloth
Sage Products LLC
----------
RestoreZ
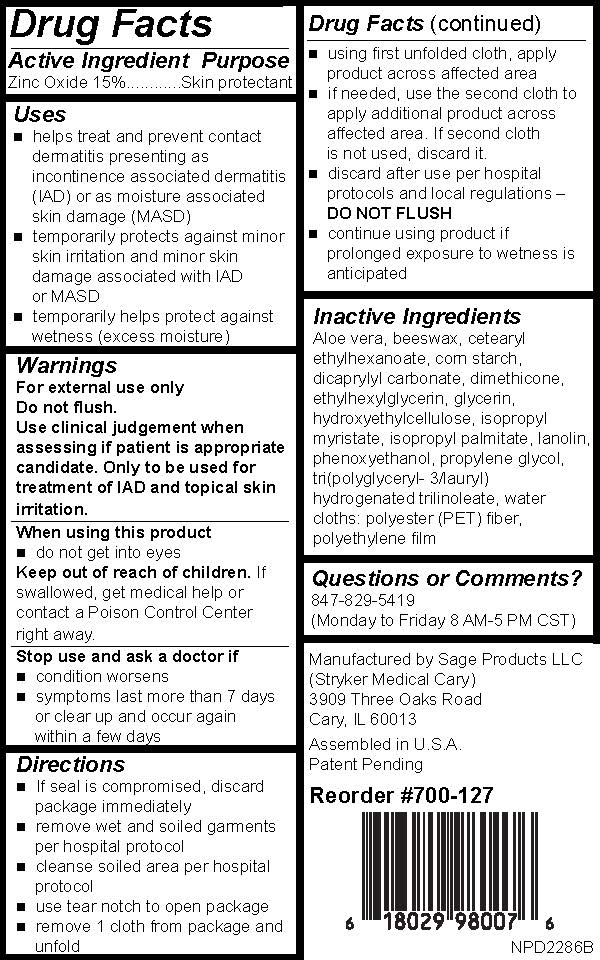
Uses
- helps treat and prevent contact dermatitis presenting as incontinence associated dermatitis (IAD) or as moisture associated skin damage (MASD)
- temporarily protects against minor skin irritation and minor skin damage associated with IAD or MASD
- temporarily helps protect against wetness (excess moisture)
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Directions
- If seal is compromised, discard package immediately
- remove wet and soiled garments per hospital protocol
- cleanse soiled area per hospital protocol
- use tear notch to open package
- remove 1 cloth from package and unfold
- using first unfolded cloth, apply product across affected area
- If needed, use the second cloth to apply additional product across affected area. If second cloth is not used, discard it.
- discard after use per hospital protocols and local regulations –DO NOT FLUSH
- continue using product if prolonged exposure to wetness is anticipated
Inactive Ingredients
Aloe vera, beeswax, cetearyl ethylhexanoate, corn starch, dicaprylyl carbonate, dimethicone, ethylhexylglycerin, glycerin, hydroxyethylcellulose, isopropyl myristate, isopropyl palmitate, lanolin, phenoxyethanol, propylene glycol, tri(polyglyceryl- 3/lauryl) hydrogenated trilinoleate, water
cloths: polyester (PET) fiber, polyethylene film
| RESTOREZ
zinc oxide cloth |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Sage Products LLC (054326178) |
Revised: 3/2024
Document Id: 12ee95f6-e4aa-2270-e063-6294a90a5b25
Set id: 70b2b3c2-f0fd-40f0-b6f2-5f8522aca161
Version: 6
Effective Time: 20240305
Trademark Results [RestoreZ]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RESTOREZ 87847476 not registered Dead/Abandoned |
Savant Science Inc. 2018-03-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.