METHOCARBAMOL TABLETS, USP, 500 MG- methocarbamol tablet, film coated METHOCARBAMOL TABLETS, USP, 750 MG- methocarbamol tablet, film coated
Methocarbamol Tablets, USP, 750 mg by
Drug Labeling and Warnings
Methocarbamol Tablets, USP, 750 mg by is a Prescription medication manufactured, distributed, or labeled by Granulation Technology, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Methocarbamol Tablets, USP, a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.
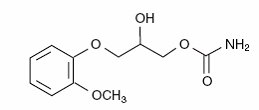
The chemical name of methocarbamol is 3-(2-methoxyphenoxy)-1,2-propanediol 1-carbamate and has the empirical formula C11H15NO5. Its molecular weight is 241.24. The structural formula is shown below.
Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and n-hexane.
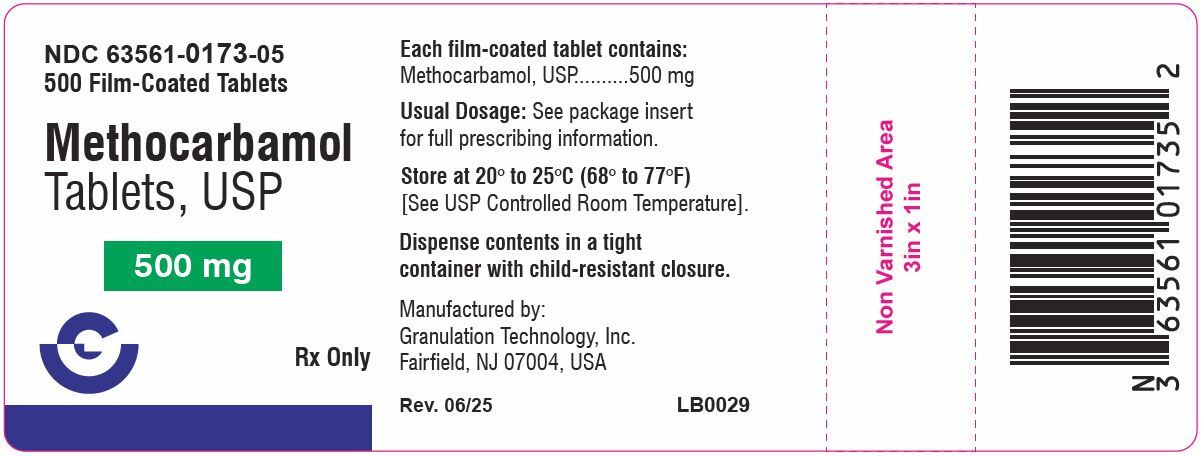
Methocarbamol Tablets, USP 500 mg are available as a white, round, scored, film-coated tablet containing 500 mg of methocarbamol, USP for oral administration. The inactive ingredients present are microcrystalline cellulose, croscarmellose sodium, povidone (k-30), sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol, polysorbate 80.
Methocarbamol Tablets, USP 750 mg are available as a white, capsule-shaped, film-coated tablet containing 750 mg of methocarbamol, USP for oral administration. The inactive ingredients present are microcrystalline cellulose, croscarmellose sodium, povidone (k-30), sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol, polysorbate 80.
-
CLINICAL PHARMACOLOGY
The mechanism of action of methocarbamol in humans has not been established, but may be due to general CNS depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
Pharmacokinetics
In healthy volunteers, the plasma clearance of methocarbamol ranges between 0.20 and 0.80 L/h/kg, the mean plasma elimination half-life ranges between 1 and 2 hours, and the plasma protein binding ranges between 46% and 50%.
Methocarbamol is metabolized via dealkylation and hydroxylation. Conjugation of methocarbamol also is likely. Essentially all methocarbamol metabolites are eliminated in the urine. Small amounts of unchanged methocarbamol also are excreted in the urine.
Special populations
Elderly
The mean (± SD) elimination half-life of methocarbamol in elderly healthy volunteers (mean [± SD] age, 69 [± 4] years) was slightly prolonged compared to a younger (mean [± SD] age, 53.3 [± 8.8] years), healthy population (1.5 [± 0.4] hours versus 1.1 [±0.27] hours, respectively). The fraction of bound methocarbamol was slightly decreased in the elderly versus younger volunteers (41% to 43% versus 46% to 50%, respectively).
Renally impaired
The clearance of methocarbamol in 8 renally-impaired patients on maintenance hemodialysis was reduced about 40% compared to 17 normal subjects, although the mean (± SD) elimination half- life in these two groups was similar: 1.2 (± 0.6) versus 1.1 (± 0.3) hours, respectively.
Hepatically impaired
In 8 patients with cirrhosis secondary to alcohol abuse, the mean total clearance of methocarbamol was reduced approximately 70% compared to that obtained in 8 age- and weight-matched normal subjects. The mean (± SD) elimination half-life in the cirrhotic patients and the normal subjects was 3.38 (± 1.62) hours and 1.11 (± 0.27) hours, respectively. The percent of methocarbamol bound to plasma proteins was decreased to approximately 40% to 45% compared to 46% to 50% in the normal subjects.
-
INDICATIONS AND USAGE
Methocarbamol Tablets, USP are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
- CONTRAINDICATIONS
-
WARNINGS
Since methocarbamol may possess a general CNS depressant effect, patients receiving Methocarbamol Tablets, USP should be cautioned about combined effects with alcohol and other CNS depressants.
Safe use of Methocarbamol Tablets, USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in uteroexposure to methocarbamol. Therefore, Methocarbamol Tablets, USP should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see PRECAUTIONS, Pregnancy).
Use in Activities Requiring Mental Alertness
Methocarbamol may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
-
PRECAUTIONS
Information for Patients
Patients should be cautioned that methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery.
Because methocarbamol may possess a general CNS-depressant effect, patients should be cautioned about combined effects with alcohol and other CNS depressants.
Drug Interactions
See WARNINGSand PRECAUTIONSfor interaction with CNS drugs and alcohol.
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Drug/Laboratory Test Interactions
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) using nitrosonaphthol reagent and in screening tests for urinary vanillylmandelic acid (VMA) using the Gitlow method.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed. No studies have been conducted to assess the effect of methocarbamol on mutagenesis or its potential to impair fertility.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with methocarbamol. It is also not known whether methocarbamol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methocarbamol Tablets, USP should be given to a pregnant woman only if clearly needed.
Safe use of Methocarbamol Tablets, USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in uteroexposure to methocarbamol. Therefore, Methocarbamol Tablets, USP should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see WARNINGS).
Nursing Mothers
Methocarbamol and/or its metabolites are excreted in the milk of dogs; however, it is not known whether methocarbamol or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Methocarbamol Tablets, USP are administered to a nursing woman.
-
ADVERSE REACTIONS
Adverse reactions reported coincident with the administration of methocarbamol include:
Body as a whole:Anaphylactic reaction, angioneurotic edema, fever, headache
Cardiovascular system:Bradycardia, flushing, hypotension, syncope, thrombophlebitis
Digestive system:Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting
Hemic and lymphatic system:Leukopenia
Immune system:Hypersensitivity reactions
Nervous system:Amnesia, confusion, diplopia, dizziness or lightheadedness, drowsiness, insomnia, mild muscular incoordination, nystagmus, sedation, seizures (including grand mal), vertigo
Skin and special senses:Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash, urticaria
-
OVERDOSAGE
Limited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following symptoms: nausea, drowsiness, blurred vision, hypotension, seizures, and coma.
In post-marketing experience, deaths have been reported with an overdose of methocarbamol alone or in the presence of other CNS depressants, alcohol or psychotropic drugs.
Treatment
Management of overdose includes symptomatic and supportive treatment. Supportive measures include maintenance of an adequate airway, monitoring urinary output and vital signs, and administration of intravenous fluids if necessary. The usefulness of hemodialysis in managing overdose is unknown.
-
DOSAGE AND ADMINISTRATION
Methocarbamol Tablets, USP, 500 mg – Adults:
Initial dosage: 3 tablets four times daily
Maintenance dosage: 2 tablets four times dailyMethocarbamol Tablets, USP, 750 mg – Adults:
Initial dosage: 2 tablets four times daily
Maintenance dosage: 1 tablet every 4 hours or 2 tablets three times dailySix grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered.) Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
-
HOW SUPPLIED
Methocarbamol Tablets, USP, 500 mg tablets are white, round, film-coated tablets, debossed "G173" on one side, plain and scored on the other side. They are supplied as follows:
Bottles of 100 NDC: 63561-0173-01
Bottles of 500 NDC: 63561-0173-05
Methocarbamol Tablets, USP, 750 mg tablets are white, capsule-shaped, film-coated tablets, debossed "G174" on one side and plain on the other side. They are supplied as follows:
Bottles of 100 NDC: 63561-0174-01
Bottles of 500 NDC: 63561-0174-05
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METHOCARBAMOL TABLETS, USP, 500 MG
methocarbamol tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63561-0173 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOCARBAMOL (UNII: 125OD7737X) (METHOCARBAMOL - UNII:125OD7737X) METHOCARBAMOL 500 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color white Score 2 pieces Shape ROUND Size 13mm Flavor Imprint Code G173 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63561-0173-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2025 2 NDC: 63561-0173-5 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212623 06/10/2025 METHOCARBAMOL TABLETS, USP, 750 MG
methocarbamol tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63561-0174 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOCARBAMOL (UNII: 125OD7737X) (METHOCARBAMOL - UNII:125OD7737X) METHOCARBAMOL 750 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color white Score no score Shape OVAL (Capsule-shaped) Size 19mm Flavor Imprint Code G174 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63561-0174-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2025 2 NDC: 63561-0174-5 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212623 06/10/2025 Labeler - Granulation Technology, Inc. (847132193) Establishment Name Address ID/FEI Business Operations Granulation Technology, Inc. 847132193 manufacture(63561-0173, 63561-0174)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.