These highlights do not include all the information needed to use RITONAVIR Tablets safely and effectively. See full prescribing information for RITONAVIR Tablets. RITONAVIR Tablets, for oral use Initial U.S. Approval: 1996
Ritonavir by
Drug Labeling and Warnings
Ritonavir by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals USA Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RITONAVIR- ritonavir tablet, film coated
Zydus Pharmaceuticals USA Inc
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RITONAVIR Tablets safely and effectively. See full prescribing information for RITONAVIR Tablets.
RITONAVIR Tablets, for oral use Initial U.S. Approval: 1996 WARNING: DRUG-DRUG INTERACTIONS LEADING TO POTENTIALLY SERIOUS AND/OR LIFE THREATENING REACTIONSSee full prescribing information for complete boxed warningCo-administration of Ritonavir with several classes of drugs including sedative hypnotics, antiarrhythmics, or ergot alkaloid preparations may result in potentially serious and/or life-threatening adverse events due to possible effects of Ritonavir on the hepatic metabolism of certain drugs. Review medications taken by patients prior to prescribing Ritonavir or when prescribing other medications to patients already taking Ritonavir. (4, 5.1) RECENT MAJOR CHANGES
INDICATIONS AND USAGERitonavir is an HIV protease inhibitor indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONSThe following have been observed in patients receiving ritonavir:
ADVERSE REACTIONSThe most frequently reported adverse drug reactions among patients receiving Ritonavir alone or in combination with other antiretroviral drugs were gastrointestinal (including diarrhea, nausea, vomiting, abdominal pain (upper and lower), neurological disturbances (including paresthesia and oral paresthesia), rash, and fatigue/asthenia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2019 |
FULL PRESCRIBING INFORMATION
WARNING: DRUG-DRUG INTERACTIONS LEADING TO POTENTIALLY SERIOUS AND/OR LIFE THREATENING REACTIONS
Co-administration of Ritonavir with several classes of drugs including sedative hypnotics, antiarrhythmics, or ergot alkaloid preparations may result in potentially serious and/or life-threatening adverse events due to possible effects of Ritonavir on the hepatic metabolism of certain drugs. Review medications taken by patients prior to prescribing Ritonavir or when prescribing other medications to patients already taking Ritonavir [see Contraindications (4), Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Recommendations
- Ritonavir must be used in combination with other antiretroviral agents.
- Ritonavir Tablets are administered orally. Ritonavir tablets should be swallowed whole, and not chewed, broken or crushed. Take Ritonavir Tablets with meals.
2.2 Dosage Recommendations in Adults
Recommended Dosage for Treatment of HIV-1:
The recommended dosage of Ritonavir Tablets is 600 mg twice daily by mouth to be taken with meals. Use of a dose titration schedule may help to reduce treatment-emergent adverse events while maintaining appropriate ritonavir plasma levels. Ritonavir Tablets should be started at no less than 300 mg twice daily and increased at 2 to 3 day intervals by 100 mg twice daily. The maximum dose of 600 mg twice daily should not be exceeded upon completion of the titration [see Dosage and Administration (2.4)].
2.3 Dosage Recommendations in Pediatric Patients
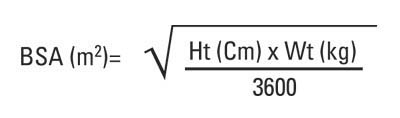
Ritonavir Tablets must be used in combination with other antiretroviral agents [see Dosage and Administration (2)]. The recommended dosage of Ritonavir Tablets in pediatric patients older than 1 month is 350 to 400 mg per m2 twice daily by mouth to be taken with meals and should not exceed 600 mg twice daily. Ritonavir Tablets should be started at 250 mg per m2 twice daily and increased at 2 to 3 day intervals by 50 mg per m2 twice daily. If patients do not tolerate 400 mg per m2 twice daily due to adverse events, the highest tolerated dose may be used for maintenance therapy in combination with other antiretroviral agents, however, alternative therapy should be considered [see Dosage and Administration (2.4)].
Body surface area (BSA) can be calculated as follows1:
2.4 Dose Modification due to Drug Interaction
Dose reduction of Ritonavir is necessary when used with other protease inhibitors: atazanavir, darunavir, fosamprenavir, saquinavir, and tipranavir.
Prescribers should consult the full prescribing information and clinical study information of these protease inhibitors if they are co-administered with a reduced dose of ritonavir [see Warnings and Precautions (5.1), and Drug Interactions (7)].
3 DOSAGE FORMS AND STRENGTHS
- Ritonavir Tablets
White film-coated ovaloid tablets debossed with the "a" logo and the code NK providing 100 mg ritonavir.
4 CONTRAINDICATIONS
- When co-administering Ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including contraindication information.
- Ritonavir is contraindicated in patients with known hypersensitivity (e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome) to ritonavir or any of its ingredients.
- Ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
- Alpha 1- Adrenoreceptor Antagonist: alfuzosin
- Antianginal: ranolazine
- Antiarrhythmics: amiodarone, dronedarone, flecainide, propafenone, quinidine
- Antifungal: voriconazole
- Anti-gout: colchicine
- Antipsychotics: lurasidone, pimozide
- Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
- GI Motility Agent: cisapride
- HMG-CoA Reductase Inhibitors: lovastatin, simvastatin
- Microsomal triglyceride transfer protein (MTTP) Inhibitor: lomitapide
- PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of pulmonary arterial hypertension
- Sedative/Hypnotics: triazolam, orally administered midazolam
- Ritonavir is contraindicated with drugs that are potent CYP3A inducers where significantly reduced ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance and cross-resistance [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
- Anticancer Agents: apalutamide
- Herbal Products: St. John's Wort (hypericum perforatum)
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving ritonavir, may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of ritonavir, respectively. These interactions may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of ritonavir.
- Loss of therapeutic effect of ritonavir and possible development of resistance.
When co-administering ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including important Warnings and Precautions.
See Table 4 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during ritonavir therapy; review concomitant medications during ritonavir therapy, and monitor for the adverse reactions associated with the concomitant medications [see Contraindications (4) and Drug Interactions (7)].
5.2 Hepatotoxicity
Hepatic transaminase elevations exceeding 5 times the upper limit of normal, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir alone or in combination with other antiretroviral drugs (see Table 4). There may be an increased risk for transaminase elevations in patients with underlying hepatitis B or C. Therefore, caution should be exercised when administering ritonavir to patients with pre-existing liver diseases, liver enzyme abnormalities, or hepatitis. Increased AST/ALT monitoring should be considered in these patients, especially during the first three months of ritonavir treatment [see Use in Specific Populations (8.6)].
There have been postmarketing reports of hepatic dysfunction, including some fatalities. These have generally occurred in patients taking multiple concomitant medications and/or with advanced AIDS.
5.3 Pancreatitis
Pancreatitis has been observed in patients receiving ritonavir therapy, including those who developed hypertriglyceridemia. In some cases fatalities have been observed. Patients with advanced HIV disease may be at increased risk of elevated triglycerides and pancreatitis [see Warnings and Precautions (5.6)]. Pancreatitis should be considered if clinical symptoms (nausea, vomiting, abdominal pain) or abnormalities in laboratory values (such as increased serum lipase or amylase values) suggestive of pancreatitis should occur. Patients who exhibit these signs or symptoms should be evaluated and ritonavir therapy should be discontinued if a diagnosis of pancreatitis is made.
5.4 Allergic Reactions/Hypersensitivity
Allergic reactions including urticaria, mild skin eruptions, bronchospasm, and angioedema have been reported. Cases of anaphylaxis, toxic epidermal necrolysis (TEN), and Stevens-Johnson syndrome have also been reported. Discontinue treatment if severe reactions develop.
5.5 PR Interval Prolongation
Ritonavir prolongs the PR interval in some patients. Post marketing cases of second or third degree atrioventricular block have been reported in patients.
Ritonavir should be used with caution in patients with underlying structural heart disease, preexisting conduction system abnormalities, ischemic heart disease, cardiomyopathies, as these patients may be at increased risk for developing cardiac conduction abnormalities.
The impact on the PR interval of co-administration of ritonavir with other drugs that prolong the PR interval (including calcium channel blockers, beta-adrenergic blockers, digoxin and atazanavir) has not been evaluated. As a result, co-administration of ritonavir with these drugs should be undertaken with caution, particularly with those drugs metabolized by CYP3A. Clinical monitoring is recommended [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
5.6 Lipid Disorders
Treatment with ritonavir therapy alone or in combination with saquinavir has resulted in substantial increases in the concentration of total cholesterol and triglycerides [see Adverse Reactions (6.1)]. Triglyceride and cholesterol testing should be performed prior to initiating ritonavir therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate, taking into account any potential drug-drug interactions with ritonavir and HMG CoA reductase inhibitors [see Contraindications (4)and Drug Interactions (7)].
5.7 Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established. Consider monitoring for hyperglycemia, new onset diabetes mellitus, or an exacerbation of diabetes mellitus in patients treated with ritonavir.
5.8 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including ritonavir. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia, or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.9 Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.10 Patients with Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced. A causal relationship between protease inhibitor therapy and these events has not been established.
5.11 Resistance/Cross-resistance
Varying degrees of cross-resistance among protease inhibitors have been observed. Continued administration of ritonavir tablets 600 mg twice daily following loss of viral suppression may increase the likelihood of cross-resistance to other protease inhibitors [see Microbiology (12.4)].
5.12 Laboratory Tests
Ritonavir has been shown to increase triglycerides, cholesterol, SGOT (AST), SGPT (ALT), GGT, CPK, and uric acid. Appropriate laboratory testing should be performed prior to initiating ritonavir therapy and at periodic intervals or if any clinical signs or symptoms occur during therapy.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling.
- Drug Interactions [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Pancreatitis [see Warnings and Precautions (5.3)]
- Allergic Reactions/Hypersensitivity [see Warnings and Precautions (5.4)]
When co-administering ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including adverse reactions.
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ritonavir alone and in combination with other antiretroviral agents was studied in 1,755 adult patients. Table 2 lists treatment-emergent Adverse Reactions (with possible or probable relationship to study drug) occurring in greater than or equal to 1% of adult patients receiving ritonavir in combined Phase II/IV studies.
The most frequently reported adverse drug reactions among patients receiving ritonavir alone or in combination with other antiretroviral drugs were gastrointestinal (including diarrhea, nausea, vomiting, abdominal pain (upper and lower)), neurological disturbances (including paresthesia and oral paresthesia), rash, and fatigue/asthenia.
| Adverse Reactions | n | % |
| Eye disorders | ||
| Blurred vision | 113 | 6.4 |
| Gastrointestinal disorders | ||
| Abdominal Pain (upper and lower)* | 464 | 26.4 |
| Diarrhea including severe with electrolyte imbalance* | 1,192 | 67.9 |
| Dyspepsia | 201 | 11.5 |
| Flatulence | 142 | 8.1 |
| Gastrointestinal hemorrhage* | 41 | 2.3 |
| Gastroesophageal reflux disease (GERD) | 19 | 1.1 |
| Nausea | 1,007 | 57.4 |
| Vomiting* | 559 | 31.9 |
| General disorders and administration site conditions | ||
| Fatigue including asthenia* | 811 | 46.2 |
| Hepatobiliary disorders | ||
| Blood bilirubin increased (including jaundice)* | 25 | 1.4 |
| Hepatitis (including increased AST, ALT, GGT)* | 153 | 8.7 |
| Immune system disorders | ||
| Hypersensitivity including urticaria and face edema* | 114 | 8.2 |
| Metabolism and nutrition disorders | ||
| Edema and peripheral edema* | 110 | 6.3 |
| Gout* | 24 | 1.4 |
| Hypercholesterolemia* | 52 | 3.0 |
| Hypertriglyceridemia* | 158 | 9.0 |
| Lipodystrophy acquired* | 51 | 2.9 |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia and back pain* | 326 | 18.6 |
| Myopathy/creatine phosphokinase increased* | 66 | 3.8 |
| Myalgia | 156 | 8.9 |
| Nervous system disorders | ||
| Dizziness* | 274 | 15.6 |
| Dysgeusia* | 285 | 16.2 |
| Paresthesia (including oral paresthesia)* | 889 | 50.7 |
| Peripheral neuropathy | 178 | 10.1 |
| Syncope* | 58 | 3.3 |
| Psychiatric disorders | ||
| Confusion* | 52 | 3.0 |
| Disturbance in attention | 44 | 2.5 |
| Renal and urinary disorders | ||
| Increased urination* | 74 | 4.2 |
| Respiratory, thoracic and mediastinal disorders | ||
| Coughing* | 380 | 21.7 |
| Oropharyngeal Pain* | 279 | 15.9 |
| Skin and subcutaneous tissue disorders | ||
| Acne* | 67 | 3.8 |
| Pruritus* | 214 | 12.2 |
| Rash (includes erythematous and maculopapular)* | 475 | 27.1 |
| Vascular disorders | ||
| Flushing, feeling hot* | 232 | 13.2 |
| Hypertension* | 58 | 3.3 |
| Hypotension including orthostatic hypotension* | 30 | 1.7 |
| Peripheral coldness* | 21 | 1.2 |
| * Represents a medical concept including several similar MedDRA PTs | ||
Laboratory Abnormalities in Adults
Table 2 shows the percentage of adult patients who developed marked laboratory abnormalities.
| Study 245
Naive Patients | Study 247
Advanced Patients | Study 462 PI-Naive Patients | |||||
| Variable | Limit | Ritonavir plus ZDV | Ritonavir | ZDV | Ritonavir | Placebo | Ritonavir plus Saquinavir |
| Chemistry | High | ||||||
| Cholesterol | > 240 mg/dL | 30.7 | 44.8 | 9.3 | 36.5 | 8.0 | 65.2 |
| CPK | > 1000 IU/L | 9.6 | 12.1 | 11.0 | 9.1 | 6.3 | 9.9 |
| GGT | > 300 IU/L | 1.8 | 5.2 | 1.7 | 19.6 | 11.3 | 9.2 |
| SGOT (AST) | > 180 IU/L | 5.3 | 9.5 | 2.5 | 6.4 | 7.0 | 7.8 |
| SGPT (ALT) | > 215 IU/L | 5.3 | 7.8 | 3.4 | 8.5 | 4.4 | 9.2 |
| Triglycerides | > 800 mg/dL | 9.6 | 17.2 | 3.4 | 33.6 | 9.4 | 23.4 |
| Triglycerides | > 1500 mg/dL | 1.8 | 2.6 | - | 12.6 | 0.4 | 11.3 |
| Triglycerides Fasting | > 1500 mg/dL | 1.5 | 1.3 | - | 9.9 | 0.3 | - |
| Uric Acid | > 12 mg/dL | - | - | - | 3.8 | 0.2 | 1.4 |
| Hematology | Low | ||||||
| Hematocrit | < 30% | 2.6 | - | 0.8 | 17.3 | 22.0 | 0.7 |
| Hemoglobin | < 8.0 g/dL | 0.9 | - | - | 3.8 | 3.9 | - |
| Neutrophils | ≤ 0.5 x 109/L | - | - | - | 6.0 | 8.3 | - |
| RBC | < 3.0 x 1012/L | 1.8 | - | 5.9 | 18.6 | 24.4 | - |
| WBC | < 2.5 x 109/L | - | 0.9 | 6.8 | 36.9 | 59.4 | 3.5 |
| - Indicates no events reported. | |||||||
Adverse Reactions in Pediatric Patients
Ritonavir has been studied in 265 pediatric patients greater than 1 month to 21 years of age. The adverse event profile observed during pediatric clinical trials was similar to that for adult patients.
Vomiting, diarrhea, and skin rash/allergy were the only drug-related clinical adverse events of moderate to severe intensity observed in greater than or equal to 2% of pediatric patients enrolled in ritonavir clinical trials.
Laboratory Abnormalities in Pediatric Patients
The following Grade 3-4 laboratory abnormalities occurred in greater than 3% of pediatric patients who received treatment with ritonavir either alone or in combination with reverse transcriptase inhibitors: neutropenia (9%), hyperamylasemia (7%), thrombocytopenia (5%), anemia (4%), and elevated AST (3%).
6.2 Postmarketing Experience
The following adverse events (not previously mentioned in the labeling) have been reported during post-marketing use of ritonavir. Because these reactions are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or establish a causal relationship to ritonavir exposure.
Dehydration, usually associated with gastrointestinal symptoms, and sometimes resulting in hypotension, syncope, or renal insufficiency has been reported. Syncope, orthostatic hypotension, and renal insufficiency have also been reported without known dehydration.
Co-administration of ritonavir with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system.
First-degree AV block, second-degree AV block, third-degree AV block, right bundle branch block have been reported [see Warnings and Precautions (5.5)].
Cardiac and neurologic events have been reported when ritonavir has been co-administered with disopyramide, mexiletine, nefazodone, fluoxetine, and beta blockers. The possibility of drug interaction cannot be excluded.
Cushing's syndrome and adrenal suppression have been reported when ritonavir has been co-administered with fluticasone propionate or budesonide.
There have been postmarketing reports of seizure. Also, see Cardiovascular System.
7 DRUG INTERACTIONS
When co-administering ritonavir with other protease inhibitors (atazanavir, darunavir, fosamprenavir, saquinavir, and tipranavir), see the full prescribing information for that protease inhibitor including important information for drug interactions.
7.1 Potential for Ritonavir to Affect Other Drugs
Ritonavir is an inhibitor of cytochrome P450 3A (CYP3A) and may increase plasma concentrations of agents that are primarily metabolized by CYP3A. Agents that are extensively metabolized by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in AUC (greater than 3-fold) when co-administered with ritonavir. Thus, co-administration of ritonavir with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. Co-administration with other CYP3A substrates may require a dose adjustment or additional monitoring as shown in Table 5.
Ritonavir also inhibits CYP2D6 to a lesser extent. Co-administration of substrates of CYP2D6 with ritonavir could result in increases (up to 2-fold) in the AUC of the other agent, possibly requiring a proportional dosage reduction. Ritonavir also appears to induce CYP3A, CYP1A2, CYP2C9, CYP2C19, and CYP2B6 as well as other enzymes, including glucuronosyl transferase.
These examples are a guide and not considered a comprehensive list of all possible drugs that may interact with ritonavir. The healthcare provider should consult appropriate references for comprehensive information.
7.2 Established and Other Potentially Significant Drug Interactions
Table 3 provides a list of established or potentially clinically significant drug interactions. Alteration in dose or regimen may be recommended based on drug interaction studies or predicted interaction [see Clinical Pharmacology (12.3)] for magnitude of interaction.
| Concomitant Drug Class: Drug Name | Effect on Concentration of Ritonavir or Concomitant Drug | Clinical Comment |
| HIV-Antiviral Agents | ||
| HIV-1 Protease Inhibitor: atazanavir darunavir fosamprenavir | ↑ amprenavir ↑ atazanavir ↑ darunavir | See the complete prescribing information for fosamprenavir, atazanavir, darunavir for details on co-administration with ritonavir. |
| HIV-1 Protease Inhibitor: indinavir | ↑ indinavir | Appropriate doses for this combination, with respect to efficacy and safety, have not been established. |
| HIV-1 Protease Inhibitor: saquinavir | ↑ saquinavir | See the complete prescribing information for saquinavir for details on co-administration of saquinavir and ritonavir. Saquinavir/ritonavir in combination with rifampin is not recommended due to the risk of severe hepatotoxicity (presenting as increased hepatic transaminases) if the three drugs are given together. |
| HIV-1 Protease Inhibitor: tipranavir | ↑ tipranavir | See the complete prescribing information for tipranavir for details on co-administration of tipranavir and ritonavir. |
| Non-Nucleoside Reverse Transcriptase Inhibitor: delavirdine | ↑ ritonavir | Appropriate doses of this combination with respect to safety and efficacy have not been established. |
| HIV-1 CCR5 – antagonist: maraviroc | ↑ maraviroc | See the complete prescribing information for maraviroc for details on co-administration of maraviroc and ritonavir-containing protease inhibitors. |
| Integrase Inhibitor: raltegravir | ↓ raltegravir | The effects of ritonavir on raltegravir with ritonavir dosage regimens greater than 100 mg twice daily have not been evaluated, however raltegravir concentrations may be decreased with ritonavir coadministration. |
| Other Agents | ||
| Alpha 1- Adrenoreceptor Antagonist: alfuzosin | ↑ alfuzosin | Contraindicated due to potential hypotension [see Contraindications (4)]. |
| Antianginal: ranolazine | ↑ ranolazine | Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. |
| Analgesics, Narcotic: tramadol, propoxyphene, methadone, fentanyl | ↑ analgesics ↓ methadone ↑ fentanyl | A dose decrease may be needed for these drugs when co-administered with ritonavir. Dosage increase of methadone may be considered. Careful monitoring of therapeutic and adverse effects (including potentially fatal respiratory depression) is recommended when fentanyl is concomitantly administered with ritonavir. |
| Anesthetic: meperidine | ↓ meperidine/ ↑ normeperidine (metabolite) | Dosage increase and long-term use of meperidine with ritonavir are not recommended due to the increased concentrations of the metabolite normeperidine which has both analgesic activity and CNS stimulant activity (e.g., seizures). |
| Antialcoholics: disulfiram/ metronidazole | Ritonavir formulations contain ethanol, which can produce disulfiram-like reactions when co-administered with disulfiram or other drugs that produce this reaction (e.g., metronidazole). | |
| Antiarrhythmics: amiodarone, dronedarone, flecainide, propafenone, quinidine | ↑ antiarrhythmics | Contraindicated due to potential for cardiac arrhythmias [see Contraindications (4)]. |
| Antiarrhythmics: disopyramide, lidocaine, mexiletine | ↑ antiarrhythmics | Caution is warranted and therapeutic concentration monitoring is recommended for antiarrhythmics when co-administered with ritonavir, if available. |
| Anticancer Agents: abemaciclib, apalutamide, dasatinib, encorafenib, ibrutinib, ivosidenib, neratinib, nilotinib, venetoclax, vinblastine, vincristine | ↑ anticancer agents ↓ ritonavir# | Apalutamide is contraindicated due to potential for loss of virologic response and possible resistance to ritonavir or to the class of protease inhibitors [see Contraindications (4)]. Avoid co-administration of encorafenib or ivosidenib with ritonavir due to potential risk of serious adverse events such as QT interval prolongation. If co-administration of encorafenib with ritonavir cannot be avoided, modify dose as recommended in encorafenib USPI. If co-administration of ivosidenib with ritonavir cannot be avoided, reduce ivosidenib dose to 250 mg once daily. Avoid use of neratinib, venetoclax or ibrutinib with ritonavir. For vincristine and vinblastine, consideration should be given to temporarily withholding the ritonavir containing antiretroviral regimen in patients who develop significant hematologic or gastrointestinal side effects when ritonavir is administered concurrently with vincristine or vinblastine. Clinicians should be aware that if the ritonavir containing regimen is withheld for a prolonged period, consideration should be given to altering the regimen to not include a CYP3A or P-gp inhibitor in order to control HIV-1 viral load. A decrease in the dosage or an adjustment of the dosing interval of nilotinib and dasatinib may be necessary for patients requiring co-administration with strong CYP3A inhibitors such as ritonavir. Please refer to the nilotinib and dasatinib prescribing information for dosing instructions. |
| Anticoagulant: warfarin | ↑↓ warfarin | Initial frequent monitoring of the INR during ritonavir and warfarin co-administration is recommended. |
| Anticoagulant: rivaroxaban | ↑ rivaroxaban | Avoid concomitant use of rivaroxaban and ritonavir. Co-administration of ritonavir and rivaroxaban may lead to risk of increased bleeding. |
| Anticonvulsants: carbamazepine, clonazepam, ethosuximide | ↑ anticonvulsants | A dose decrease may be needed for these drugs when co-administered with ritonavir and therapeutic concentration monitoring is recommended for these anticonvulsants, if available. |
| Anticonvulsants: divalproex, lamotrigine, phenytoin | ↓ anticonvulsants | A dose increase may be needed for these drugs when co-administered with ritonavir and therapeutic concentration monitoring is recommended for these anticonvulsants, if available. |
| Antidepressants: nefazodone, selective serotonin reuptake inhibitors (SSRIs): e.g. fluoxetine, paroxetine, tricyclics: e.g. amitriptyline, nortriptyline | ↑ antidepressants | A dose decrease may be needed for these drugs when co-administered with ritonavir. |
| Antidepressant: bupropion | ↓ bupropion ↓ active metabolite, hydroxybupropion | Patients receiving ritonavir and bupropion concurrently should be monitored for an adequate clinical response to bupropion. |
| Antidepressant: desipramine | ↑ desipramine | Dosage reduction and concentration monitoring of desipramine is recommended. |
| Antidepressant: trazodone | ↑ trazodone | Adverse events of nausea, dizziness, hypotension and syncope have been observed following co-administration of trazodone and ritonavir. A lower dose of trazodone should be considered. |
| Antiemetic: dronabinol | ↑ dronabinol | A dose decrease of dronabinol may be needed when co-administered with ritonavir. |
| Antifungals: ketoconazole itraconazole voriconazole | ↑ ketoconazole ↑ itraconazole ↓ voriconazole | High doses of ketoconazole or itraconazole (greater than 200 mg per day) are not recommended. Co-administration of voriconazole and ritonavir doses of 400 mg every 12 hours or greater is contraindicated due to the potential for loss of antifungal response [see Contraindications (4)]. Co-administration of voriconazole and ritonavir 100 mg should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole. |
| Anti-gout: colchicine | ↑ colchicine | Contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see Contraindications (4)]. For patients with normal renal or hepatic function: Treatment of gout flares-co-administration of colchicine in patients on ritonavir: 0.6 mg (one tablet) for one dose, followed by 0.3 mg (half tablet) one hour later. Dose to be repeated no earlier than three days. Prophylaxis of gout flares-co-administration of colchicine in patients on ritonavir: If the original colchicine regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original colchicine regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. Treatment of familial Mediterranean fever (FMF)-co-administration of colchicine in patients on ritonavir: Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day). |
| Anti-infective: clarithromycin | ↑ clarithromycin | For patients with renal impairment, adjust clarithromycin dose as follows:
|
| Antimycobacterial: bedaquiline | ↑ bedaquiline | Bedaquiline should only be used with ritonavir if the benefit of co-administration outweighs the risk. |
| Antimycobacterial: rifabutin | ↑ rifabutin and rifabutin metabolite | Dosage reduction of rifabutin by at least three-quarters of the usual dose of 300 mg per day is recommended (e.g., 150 mg every other day or three times a week). Further dosage reduction may be necessary. |
| Antimycobacterial: rifampin | ↓ ritonavir | May lead to loss of virologic response. Alternate antimycobacterial agents such as rifabutin should be considered. |
| Antiparasitic: atovaquone | ↓ atovaquone | Clinical significance is unknown; however, increase in atovaquone dose may be needed. |
| Antiparasitic: quinine | ↑ quinine | A dose decrease of quinine may be needed when co-administered with ritonavir. |
| Antipsychotics: lurasidone pimozide | ↑ lurasidone ↑ pimozide | Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| Antipsychotics: perphenazine, risperidone, thioridazine | ↑ antipsychotics | A dose decrease may be needed for these drugs when co-administered with ritonavir. |
| Antipsychotics: quetiapine | ↑ quetiapine | Initiation of ritonavir in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. Initiation of quetiapine in patients taking ritonavir: Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine. |
| β-Blockers: metoprolol, timolol | ↑ beta-blockers | Caution is warranted and clinical monitoring of patients is recommended. A dose decrease may be needed for these drugs when co-administered with ritonavir. |
| Bronchodilator: theophylline | ↓ theophylline | Increased dosage of theophylline may be required; therapeutic monitoring should be considered. |
| Calcium channel blockers: diltiazem, nifedipine, verapamil | ↑ calcium channel blockers | Caution is warranted and clinical monitoring of patients is recommended. A dose decrease may be needed for these drugs when co-administered with ritonavir. |
| Digoxin | ↑ digoxin | Concomitant administration of ritonavir with digoxin may increase digoxin levels. Caution should be exercised when co-administering ritonavir with digoxin, with appropriate monitoring of serum digoxin levels. |
| Endothelin receptor antagonists: bosentan | ↑ bosentan | Co-administration of bosentan in patients on ritonavir:
In patients who have been receiving ritonavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability. Co-administration of ritonavir in patients on bosentan: Discontinue use of bosentan at least 36 hours prior to initiation of ritonavir. After at least 10 days following the initiation of ritonavir, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability. |
| GnRH Receptor Antagonists: elagolix | ↑ elagolix ↓ ritonavir | Concomitant use of elagolix 200 mg twice daily and ritonavir for more than 1 month is not recommended due to potential risk of adverse events such as bone loss and hepatic transaminase elevations. Limit concomitant use of elagolix 150 mg once daily and ritonavir to 6 months. |
| Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine | ↑ ergot derivatives | Contraindicated due to potential for acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system [see Contraindications (4)]. |
| GI Motility Agent: cisapride | ↑ cisapride | Contraindicated due to potential for cardiac arrhythmias [see Contraindications (4)]. |
| Hepatitis C direct acting antiviral: glecaprevir/pibrentasvir simeprevir |
↑ glecaprevir ↑ pibrentasvir ↑simeprevir | It is not recommended to co-administer ritonavir with glecaprevir/pibrentasvir, or simeprevir. |
| Herbal Products: St. John's Wort (hypericum perforatum) | ↓ ritonavir | Contraindicated due to potential for loss of virologic response and possible resistance to NORVIR or to the class of protease inhibitors [see Contraindications (4)]. |
| Lipid-modifying agents HMG-CoA Reductase Inhibitor: lovastatin simvastatin atorvastatin rosuvastatin Microsomal triglyceride transfer protein (MTTP) Inhibitor: lomitapide |
↑ lovastatin ↑ simvastatin ↑ atorvastatin ↑ rosuvastatin ↑ lomitapide |
Contraindicated due to potential for myopathy including rhabdomyolysis [see Contraindications (4)]. Titrate atorvastatin and rosuvastatin dose carefully and use the lowest necessary dose. If ritonavir is used with another protease inhibitor, see the complete prescribing information for the concomitant protease inhibitor for details on co-administration with atorvastatin and rosuvastatin. Lomitapide is a sensitive substrate for CYP3A4 metabolism. CYP3A4 inhibitors increase the exposure of lomitapide, with strong inhibitors increasing exposure approximately 27-fold. Concomitant use of moderate or strong CYP3A4 inhibitors with lomitapide is contraindicated due to potential for hepatotoxicity [see Contraindications (4)]. |
| Immunosuppressants: cyclosporine, tacrolimus, sirolimus (rapamycin) | ↑ immunosuppressants | Therapeutic concentration monitoring is recommended for immunosuppressant agents when co-administered with ritonavir. |
| Kinase Inhibitors: fostamatinib (also see anticancer agents above) | ↑ fostamatinib metabolite R406 | Monitor for toxicities of R406 exposure resulting in dose-related adverse events such as hepatotoxicity and neutropenia. Fostamatinib dose reduction may be required. |
| Long-acting beta-adrenoceptor agonist: salmeterol | ↑ salmeterol | Concurrent administration of salmeterol and ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. |
| Oral Contraceptives or Patch Contraceptives: ethinyl estradiol | ↓ ethinyl estradiol | Alternate methods of contraception should be considered. |
| PDE5 Inhibitors: avanafil sildenafil, tadalafil, vardenafil | ↑ avanafil ↑ sildenafil ↑ tadalafil ↑ vardenafil | Sildenafil when used for the treatment of pulmonary arterial hypertension (Revatio®) is contraindicated due to the potential for sildenafil-associated adverse events, including visual abnormalities, hypotension, prolonged erection, and syncope [see Contraindications (4)]. Do not use ritonavir with avanafil because a safe and effective avanafil dosage regimen has not been established. Particular caution should be used when prescribing sildenafil, tadalafil or vardenafil in patients receiving ritonavir. Coadministration of ritonavir with these drugs may result in an increase in PDE5 inhibitor associated adverse events, including hypotension, syncope, visual changes, and prolonged erection. Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH): Sildenafil (Revatio®) is contraindicated [see Contraindications (4)]. The following dose adjustments are recommended for use of tadalafil (Adcirca®) with ritonavir: Co-administration of ADCIRCA in patients on ritonavir: In patients receiving ritonavir for at least one week, start ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability. Co-administration of ritonavir in patients on ADCIRCA: Avoid use of ADCIRCA during the initiation of ritonavir. Stop ADCIRCA at least 24 hours prior to starting ritonavir. After at least one week following the initiation of ritonavir, resume ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability. Use of PDE5 inhibitors for the treatment of erectile dysfunction: It is recommended not to exceed the following doses:
Use with increased monitoring for adverse events. |
| Sedative/hypnotics: buspirone, clorazepate, diazepam, estazolam, flurazepam, zolpidem | ↑ sedative/hypnotics | A dose decrease may be needed for these drugs when co-administered with ritonavir. |
| Sedative/Hypnotics: triazolam, orally administered midazolam | ↑ triazolam ↑ midazolam | Contraindicated due to potential for prolonged or increased sedation or respiratory depression [see Contraindications (4)]. |
| Sedative/hypnotics: Parenteral midazolam | ↑ midazolam | Co-administration should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. |
| Stimulant: methamphetamine | ↑ methamphetamine | Use with caution. A dose decrease of methamphetamine may be needed when co-administered with ritonavir. |
| Systemic/Inhaled/ Nasal/Ophthalmic Corticosteroids: e.g., betamethasone budesonide ciclesonide dexamethasone fluticasone methylprednisolone mometasone prednisone triamcinolone | ↑ glucocorticoids | Coadministration with corticosteroids whose exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing’s syndrome and adrenal suppression. Alternative corticosteroids including beclomethasone and prednisolone (whose PK and/or PD are less affected by strong CYP3A inhibitors relative to other studied steroids) should be considered, particularly for long-term use. |
| # refers to interaction with apalutamide. | ||
8 USE IN SPECIFIC POPULATIONS
When co-administering ritonavir with other protease inhibitors, see the full prescribing information for the co-administered protease inhibitor including important information for use in special populations.
8.1 Pregnancy
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ritonavir during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Prospective pregnancy data from the Antiretroviral Pregnancy Registry (APR) are not sufficient to adequately assess the risk of birth defects or miscarriage. Available data from the APR show no difference in the rate of overall birth defects for ritonavir compared to the background rate for major birth defects of 2.7% in the U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) [see Data].
In animal reproduction studies, no evidence of adverse developmental outcomes was observed with oral administration of ritonavir to pregnant rats and rabbits. During organogenesis in the rat and rabbit, systemic exposure (AUC) was approximately 1/3 lower than human exposure at the recommended daily dose. In the rat pre- and post-natal developmental study, maternal systemic exposure to ritonavir was approximately 1/2 of the exposure in humans at the recommended daily dose, based on a body surface area conversion factor [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Based on prospective reports to the APR of approximately 6100 live births following exposure to ritonavir-containing regimens (including over 2800 live births exposed in the first trimester and over 3200 live births exposed in the second and third trimesters), there was no difference in the rate of overall birth defects for ritonavir compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of birth defects in live births was 2.3% (95% CI: 1.7%-2.9%) following first-trimester exposure to ritonavir-containing regimens and 2.9% (95% CI: 2.3%-3.5%) following second and third trimester exposure to ritonavir-containing regimens.
While placental transfer of ritonavir and fetal ritonavir concentrations are generally low, detectable levels have been observed in cord blood samples and neonate hair.
Ritonavir was administered orally to pregnant rats (at 0, 15, 35, and 75 mg/kg/day) and rabbits (at 0, 25, 50, and 110 mg/kg/day) during organogenesis (on gestation days 6 through 17 and 6 through 19, respectively). No evidence of teratogenicity due to ritonavir was observed in rats and rabbits at doses producing systemic exposures (AUC) equivalent to approximately 1/3 lower than human exposure at the recommended daily dose. Developmental toxicity observed in rats (early resorptions, decreased fetal body weight and ossification delays and developmental variations) occurred at a maternally toxic dose, at an exposure equivalent to approximately 1/3 lower than human exposure at the recommended daily dose. A slight increase in the incidence of cryptorchidism was also noted in rats (at a maternally toxic dose) at an exposure approximately 1/5 lower than human exposure at the recommended daily dose. Developmental toxicity was observed in rabbits (resorptions, decreased litter size and decreased fetal weights) at maternally toxic doses approximately 1.8 times higher than the recommended daily dose, based on a body surface area conversion factor. In pre-and postnatal development study in rats, ritonavir was administered at doses of 0, 15, 35, and 60 mg/kg/day from gestation day 6 through postnatal day 20. At doses of 60 mg/kg/day, no developmental toxicity was noted with ritonavir dosage equivalent to 1/2 of the recommended daily dose, based on a body surface area conversion factor.
8.2 Lactation
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
Limited published data reports that ritonavir is present in human milk.
There is no information on the effects of ritonavir on the breastfed infant or the effects of the drug on milk production. Because of the potential for (1) HIV transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants) and (3) serious adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving ritonavir.
8.3 Females and Males of Reproductive Potential
Use of ritonavir may reduce the efficacy of combined hormonal contraceptives. Advise patients using combined hormonal contraceptives to use an effective alternative contraceptive method or an additional barrier method of contraception [see Drug Interactions (7.2)].
8.4 Pediatric Use
In HIV-infected patients age greater than 1 month to 21 years, the antiviral activity and adverse event profile seen during clinical trials and through postmarketing experience were similar to that for adult patients.
8.5 Geriatric Use
Clinical studies of ritonavir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
No dose adjustment of ritonavir is necessary for patients with either mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of ritonavir in subjects with severe hepatic impairment (Child-Pugh Class C), therefore, ritonavir is not recommended for use in patients with severe hepatic impairment [see Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Acute Overdosage - Human Overdose Experience
Human experience of acute overdose with ritonavir is limited. One patient in clinical trials took ritonavir 1500 mg per day for two days. The patient reported paresthesias which resolved after the dose was decreased. A post-marketing case of renal failure with eosinophilia has been reported with ritonavir overdose.
The approximate lethal dose was found to be greater than 20 times the related human dose in rats and 10 times the related human dose in mice.
Treatment of overdose with ritonavir consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with ritonavir. If indicated, elimination of unabsorbed drug should be achieved by gastric lavage; usual precautions should be observed to maintain the airway. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since ritonavir is extensively metabolized by the liver and is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the drug. A Certified Poison Control Center should be consulted for up-to-date information on the management of overdose with ritonavir.
11 DESCRIPTION
Ritonavir is an inhibitor of HIV protease with activity against the Human Immunodeficiency Virus (HIV).
Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12- tetraazatridecan-13-oic acid, 5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C37H48N6O5S2, and its molecular weight is 720.95. Ritonavir has the following structural formula:
Ritonavir is a white-to-light-tan powder. Ritonavir has a bitter metallic taste. It is freely soluble in methanol and ethanol, soluble in isopropanol and practically insoluble in water.
Ritonavir Tablets are available for oral administration in a strength of 100 mg ritonavir with the following inactive ingredients: copovidone, anhydrous dibasic calcium phosphate, sorbitan monolaurate, colloidal silicon dioxide, and sodium stearyl fumarate. The following are the ingredients in the film coating: hypromellose, titanium dioxide, polyethylene glycol 400, hydroxypropyl cellulose, talc, polyethylene glycol 3350, colloidal silicon dioxide, and polysorbate 80.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once-daily) controlled crossover study in 45 healthy adults, with 10 measurements over 12 hours on Day 3. The maximum mean (95% upper confidence bound) time-matched difference in QTcF from placebo after baseline correction was 5.5 (7.6) milliseconds (msec) for 400 mg twice-daily ritonavir. Ritonavir 400 mg twice daily resulted in Day 3 ritonavir exposure that was approximately 1.5 fold higher than observed with ritonavir 600 mg twice-daily dose at steady state.
PR interval prolongation was also noted in subjects receiving ritonavir in the same study on Day 3. The maximum mean (95% confidence interval) difference from placebo in the PR interval after baseline correction was 22 (25) msec for 400 mg twice-daily ritonavir [see Warnings and Precautions (5.5)].
12.3 Pharmacokinetics
The pharmacokinetics of ritonavir have been studied in healthy volunteers and HIV-infected patients (CD4 greater than or equal to 50 cells per μL). See Table 5 for ritonavir pharmacokinetic characteristics.
The absolute bioavailability of ritonavir has not been determined. Ritonavir Tablets are not bioequivalent to ritonavir capsules under moderate fat conditions (857 kcal; 31% fat, 13% protein, 56% carbohydrates). No information is available comparing ritonavir tablets to ritonavir capsules under fasting conditions.
Effect of Food on Oral Absorption
The bioavailability of Ritonavir Tablets are decreased under fed conditions as compared to fasted conditions.
Following the administration of a 100 mg tablet dose of ritonavir, Cmax and AUCinf of ritonavir were decreased by 21-23% under moderate fat (857 Kcal, 30% from fat) or high fat conditions (917 Kcal, 60% calories from fat) relative to fasting conditions.
Nearly all of the plasma radioactivity after a single oral 600 mg dose of 14C-ritonavir oral solution (n = 5) was attributed to unchanged ritonavir. Five ritonavir metabolites have been identified in human urine and feces. The isopropylthiazole oxidation metabolite (M-2) is the major metabolite and has antiviral activity similar to that of parent drug; however, the concentrations of this metabolite in plasma are low. In vitro studies utilizing human liver microsomes have demonstrated that cytochrome P450 3A (CYP3A) is the major isoform involved in ritonavir metabolism, although CYP2D6 also contributes to the formation of M–2.
In a study of five subjects receiving a 600 mg dose of 14C-ritonavir oral solution, 11.3 ± 2.8% of the dose was excreted into the urine, with 3.5 ± 1.8% of the dose excreted as unchanged parent drug. In that study, 86.4 ± 2.9% of the dose was excreted in the feces with 33.8 ± 10.8% of the dose excreted as unchanged parent drug. Upon multiple dosing, ritonavir accumulation is less than predicted from a single dose possibly due to a time and dose-related increase in clearance.
| Parameter | N | Values (Mean ± SD) |
| Vβ/F‡ | 91 | 0.41 ± 0.25 L/kg |
| t½ | 3 - 5 h | |
| CL/F SS† | 10 | 8.8 ± 3.2 L/h |
| CL/F‡ | 91 | 4.6 ± 1.6 L/h |
| CLR | 62 | < 0.1 L/h |
| RBC/Plasma Ratio | 0.14 | |
| Percent Bound* | 98 to 99% | |
| † SS = steady state; patients taking ritonavir 600 mg q12h. ‡ Single ritonavir 600 mg dose. * Primarily bound to human serum albumin and alpha-1 acid glycoprotein over the ritonavir concentration range of 0.01 to 30 µg/mL. |
||
No age-related pharmacokinetic differences have been observed in adult patients (18 to 63 years). Ritonavir pharmacokinetics have not been studied in older patients.
A study of ritonavir pharmacokinetics in healthy males and females showed no statistically significant differences in the pharmacokinetics of ritonavir. Pharmacokinetic differences due to race have not been identified.
Steady-state pharmacokinetics were evaluated in 37 HIV-infected patients ages 2 to 14 years receiving doses ranging from 250 mg per m2 twice-daily to 400 mg per m2 twice-daily in PACTG Study 310, and in 41 HIV-infected patients ages 1 month to 2 years at doses of 350 and 450 mg per m2 twice-daily in PACTG Study 345. Across dose groups, ritonavir steady-state oral clearance (CL/F/m2) was approximately 1.5 to 1.7 times faster in pediatric patients than in adult subjects. Ritonavir concentrations obtained after 350 to 400 mg per m2 twice-daily in pediatric patients greater than 2 years were comparable to those obtained in adults receiving 600 mg (approximately 330 mg per m2) twice-daily. The following observations were seen regarding ritonavir concentrations after administration with 350 or 450 mg per m2 twice-daily in children less than 2 years of age. Higher ritonavir exposures were not evident with 450 mg per m2 twice-daily compared to the 350 mg per m2 twice-daily. Ritonavir trough concentrations were somewhat lower than those obtained in adults receiving 600 mg twice-daily. The area under the ritonavir plasma concentration time curve and trough concentrations obtained after administration with 350 or 450 mg per m2 twice-daily in children less than 2 years were approximately 16% and 60% lower, respectively, than that obtained in adults receiving 600 mg twice daily.
Ritonavir pharmacokinetics have not been studied in patients with renal impairment, however, since renal clearance is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Dose-normalized steady-state ritonavir concentrations in subjects with mild hepatic impairment (400 mg twice-daily, n = 6) were similar to those in control subjects dosed with 500 mg twice-daily. Dose-normalized steady-state ritonavir exposures in subjects with moderate hepatic impairment (400 mg twice-daily, n= 6) were about 40% lower than those in subjects with normal hepatic function (500 mg twice-daily, n = 6). Protein binding of ritonavir was not statistically significantly affected by mild or moderately impaired hepatic function. No dose adjustment is recommended in patients with mild or moderate hepatic impairment. However, health care providers should be aware of the potential for lower ritonavir concentrations in patients with moderate hepatic impairment and should monitor patient response carefully. Ritonavir has not been studied in patients with severe hepatic impairment.
Based on evaluation of the published literature, ritonavir exposures are reduced during pregnancy relative to postpartum.
[see also Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7)]
Table 5 and Table 6 summarize the effects on AUC and Cmax, with 95% confidence intervals (95% CI), of co-administration of ritonavir with a variety of drugs. For information about clinical recommendations see Table 3 in Drug Interactions (7).
| Co- administered Drug | Dose of Co- administered Drug (mg) | Dose of Ritonavir (mg) | N | AUC % (95% CI) | Cmax
(95% CI) | Cmin
(95% CI) |
| Clarithromycin | 500 q12h, 4 d | 200 q8h, 4 d | 22 | ↑ 12% (2, 23%) | ↑ 15% (2, 28%) | ↑ 14% (-3, 36%) |
| Didanosine | 200 q12h, 4 d | 600 q12h, 4 d | 12 | ↔ | ↔ | ↔ |
| Fluconazole | 400 single dose, day 1; 200 daily, 4 d | 200 q6h, 4 d | 8 | ↑ 12% (5, 20%) | ↑ 15% (7, 22%) | ↑ 14% (0, 26%) |
| Fluoxetine | 30 q12h, 8 d | 600 single dose, 1 d | 16 | ↑ 19% (7, 34%) | ↔ | ND |
| Ketoconazole | 200 daily, 7 d | 500 q12h, 10 d | 12 | ↑ 18% (-3, 52%) | ↑ 10% (-11, 36%) | ND |
| Rifampin | 600 or 300 daily, 10 d | 500 q12h, 20 d | 7, 9* | ↓ 35% (7, 55%) | ↓ 25% (-5, 46%) | ↓ 49% (-14, 91%) |
| Voriconazole | 400 q12h, 1 d; then 200 q12h, 8 d | 400 q12h, 9 d | ↔ | ↔ | ND | |
| Zidovudine | 200 q8h, 4 d | 300 q6h, 4 d | 10 | ↔ | ↔ | ↔ |
| ND=not determined | ||||||
| Co- administered Drug | Dose of Co- administered Drug (mg) | Dose of Ritonavir (mg) | N | AUC % (95% CI) | Cmax
(95% CI) | Cmin
(95% CI) |
| Alprazolam | 1, single dose | 500 q12h, 10 d | 12 | ↓ 12% (-5, 30%) | ↓ 16% (5, 27%) | ND |
| Avanafil | 50, single dose | 600 q12h | 146 | ↑ 13-fold | ↑ 2.4-fold | ND |
| Clarithromycin 14-OH clarithromycin metabolite | 500 q12h, 4 d | 200 q8h, 4 d | 22 | ↑ 77% (56, 103%) ↓ 100% | ↑ 31% (15, 51%) ↓ 99% | ↑ 2.8-fold (2.4, 3.3X) ↓ 100% |
| Desipramine 2-OH desipramine metabolite | 100, single dose | 500 q12h, 12 d | 14 | ↑ 145% (103, 211%) ↓ 15% (3, 26%) | ↑ 22% (12, 35%) ↓ 67% (62, 72%) | ND ND |
| Didanosine | 200 q12h, 4 d | 600 q12h, 4 d | 12 | ↓ 13% (0, 23%) | ↓ 16% (5, 26%) | ↔ |
| Ethinyl estradiol | 50 µg single dose | 500 q12h, 16 d | 23 | ↓ 40% (31, 49%) | ↓ 32% (24, 39%) | ND |
| Fluticasone propionate aqueous nasal spray | 200 mcg qd, 7 d | 100 mg q12h, 7 d | 18 | ↑ approximately 350-fold5 | ↑ approximately 25-fold5 | |
| Indinavir1
Day 14 Day 15 | 400 q12h, 15 d | 400 q12h, 15 d | 10 |
↑ 6% (-14, 29%) ↓ 7% (-22, 28%) |
↓ 51% (40, 61%) ↓ 62% (52, 70%) | ↑ 4-fold (2.8, 6.8X) ↑ 4-fold (2.5, 6.5X) |
| Ketoconazole | 200 daily, 7 d | 500 q12h, 10 d | 12 | ↑ 3.4-fold (2.8, 4.3X) | ↑ 55% (40, 72%) | ND |
| Meperidine Normeperidine metabolite | 50 oral single dose | 500 q12h, 10 d | 8 6 | ↓ 62% (59, 65%) ↑ 47% (-24, 345%) | ↓ 59% (42, 72%) ↑ 87% (42, 147%) | ND ND |
| Methadone2 | 5, single dose | 500 q12h, 15 d | 11 | ↓ 36% (16, 52%) | ↓ 38% (28, 46%) | ND |
| Raltegravir | 400, single dose | 100 q12h, 16 d | 10 | ↓ 16% (-30, 1%) | ↓ 24% (-45, 4%) | ↓ 1% (-30, 40%) |
| Rivaroxaban | 10, single dose (days 0 and 7) | 600 q12h (days 2 to 7) | 12 | ↑ 150% (130-170%)7 | ↑ 60% (40-70%)7 | ND |
| Rifabutin 25-O-desacetyl rifabutin metabolite | 150 daily, 16 d | 500 q12h, 10 d | 5, 11* | ↑ 4-fold (2.8, 6.1X) ↑ 38-fold (28, 56X) | ↑ 2.5-fold (1.9, 3.4X) ↑ 16-fold (13, 20X) | ↑ 6-fold (3.5, 18.3X) ↑ 181-fold (ND) |
| Sildenafil | 100, single dose | 500 twice daily, 8 d | 28 | ↑ 11-fold | ↑ 4-fold | ND |
| Simeprevir | 200 mg qd, 7 d | 100 mg bid, 15 d | 12 | ↑ 618% (463%-815%)8 | ↑370% (284%-476%)8 | ↑1335% (929%-1901%)8 |
| Sulfamethoxazole3 | 800, single dose | 500 q12h, 12 d | 15 | ↓ 20% (16, 23%) | ↔ | ND |
| Tadalafil | 20 mg, single dose | 200 mg q12h | ↑ 124% | ↔ | ND | |
| Theophylline | 3 mg/kg q8h, 15 d | 500 q12h, 10 d | 13, 11* | ↓ 43% (42, 45%) | ↓ 32% (29, 34%) | ↓ 57% (55, 59%) |
| Trazodone | 50 mg, single dose | 200 mg q12h, 4 doses | 10 | ↑ 2.4-fold | ↑ 34% | |
| Trimethoprim3 | 160, single dose | 500 q12h, 12 d | 15 | ↑ 20% (3, 43%) | ↔ | ND |
| Vardenafil | 5 mg | 600 q12h | ↑ 49-fold | ↑ 13-fold | ND | |
| Voriconazole | 400 q12h, 1 d; then 200 q12h, 8 d | 400 q12h, 9 d | ↓ 82% | ↓ 66% | ||
| 400 q12h, 1 d; then 200 q12h, 8 d | 100 q12h, 9 d | ↓ 39% | ↓ 24% | |||
| Warfarin S-Warfarin R-Warfarin | 5, single dose | 400 q12h, 12d | 12 | ↑ 9% (-17, 44%)4 ↓ 33% (-38, -27%)4 | ↓ 9% (-16, -2%)4 ↔ | ND ND |
| Zidovudine | 200 q8h, 4 d | 300 q6h, 4 d | 9 | ↓ 25% (15, 34%) | ↓ 27% (4, 45%) | ND |
| ND=not determined 1 Ritonavir and indinavir were co-administered for 15 days; Day 14 doses were administered after a 15%-fat breakfast (757 Kcal) and 9%-fat evening snack (236 Kcal), and Day 15 doses were administered after a 15%-fat breakfast (757 Kcal) and 32%-fat dinner (815 Kcal). Indinavir Cmin was also increased 4-fold. Effects were assessed relative to an indinavir 800 mg q8h regimen under fasting conditions. 2 Effects were assessed on a dose-normalized comparison to a methadone 20 mg single dose. 3 Sulfamethoxazole and trimethoprim taken as single combination tablet. 4 90% CI presented for R- and S-warfarin AUC and Cmax ratios. 5 This significant increase in plasma fluticasone propionate exposure resulted in a significant decrease (86%) in plasma cortisol AUC. 6 For the reference arm: N=14 for Cmax and AUC(0-inf), and for the test arm: N=13 for Cmax and N=4 for AUC(0-inf). 7 90% CI presented for rivaroxaban 8 90% CI presented for simeprevir (change in exposure presented as percentage increase) ↑ Indicates increase, ↓ indicates decrease, ↔ indicates no change. * Parallel group design; entries are subjects receiving combination and control regimens, respectively. |
||||||
12.4 Microbiology
Ritonavir is a peptidomimetic inhibitor of the HIV-1 protease. Inhibition of HIV protease renders the enzyme incapable of processing the Gag-Pol polyprotein precursor which leads to production of non-infectious immature HIV particles.
Antiviral Activity in Cell Culture
The activity of ritonavir was assessed in acutely infected lymphoblastoid cell lines and in peripheral blood lymphocytes. The concentration of drug that inhibits 50% (EC50) value of viral replication ranged from 3.8 to 153 nM depending upon the HIV-1 isolate and the cells employed. The average EC50 value for low passage clinical isolates was 22 nM (n = 13). In MT4 cells, ritonavir demonstrated additive effects against HIV-1 in combination with either didanosine (ddI) or zidovudine (ZDV). Studies which measured cytotoxicity of ritonavir on several cell lines showed that greater than 20 microM was required to inhibit cellular growth by 50% resulting in a cell culture therapeutic index of at least 1000.
HIV-1 isolates with reduced susceptibility to ritonavir have been selected in cell culture. Genotypic analysis of these isolates showed mutations in the HIV-1 protease gene leading to amino acid substitutions I84V, V82F, A71V, and M46I. Phenotypic (n = 18) and genotypic (n = 48) changes in HIV-1 isolates from selected patients treated with ritonavir were monitored in phase I/II trials over a period of 3 to 32 weeks. Substitutions associated with the HIV–1 viral protease in isolates obtained from 43 patients appeared to occur in a stepwise and ordered fashion at positions V82A/F/T/S, I54V, A71V/T, and I36L, followed by combinations of substitutions at an additional 5 specific amino acid positions (M46I/L, K20R, I84V, L33F and L90M). Of 18 patients for whom both phenotypic and genotypic analysis were performed on free virus isolated from plasma, 12 showed reduced susceptibility to ritonavir in cell culture. All 18 patients possessed one or more substitutions in the viral protease gene. The V82A/F substitution appeared to be necessary but not sufficient to confer phenotypic resistance. Phenotypic resistance was defined as a greater than or equal to 5-fold decrease in viral sensitivity in cell culture from baseline.
Cross-Resistance to Other Antiretrovirals
Among protease inhibitors variable cross-resistance has been recognized. Serial HIV-1 isolates obtained from six patients during ritonavir therapy showed a decrease in ritonavir susceptibility in cell culture but did not demonstrate a concordant decrease in susceptibility to saquinavir in cell culture when compared to matched baseline isolates. However, isolates from two of these patients demonstrated decreased susceptibility to indinavir in cell culture (8-fold). Isolates from 5 patients were also tested for cross-resistance to amprenavir and nelfinavir; isolates from 3 patients had a decrease in susceptibility to nelfinavir (6- to 14-fold), and none to amprenavir. Cross-resistance between ritonavir and reverse transcriptase inhibitors is unlikely because of the different enzyme targets involved. One ZDV-resistant HIV-1 isolate tested in cell culture retained full susceptibility to ritonavir.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in mice and rats have been carried out on ritonavir. In male mice, at levels of 50, 100 or 200 mg per kg per day, there was a dose dependent increase in the incidence of both adenomas and combined adenomas and carcinomas in the liver. Based on AUC measurements, the exposure at the high dose was approximately 0.3-fold for males that of the exposure in humans with the recommended therapeutic dose (600 mg twice-daily). There were no carcinogenic effects seen in females at the dosages tested. The exposure at the high dose was approximately 0.6-fold for the females that of the exposure in humans. In rats dosed at levels of 7, 15 or 30 mg per kg per day there were no carcinogenic effects. In this study, the exposure at the high dose was approximately 6% that of the exposure in humans with the recommended therapeutic dose. Based on the exposures achieved in the animal studies, the significance of the observed effects is not known.
However, ritonavir was found to be negative for mutagenic or clastogenic activity in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, the mouse lymphoma assay, the mouse micronucleus test and chromosomal aberration assays in human lymphocytes.
Ritonavir produced no effects on fertility in rats at drug exposures approximately 40% (male) and 60% (female) of that achieved with the proposed therapeutic dose. Higher dosages were not feasible due to hepatic toxicity.
14 CLINICAL STUDIES
The activity of ritonavir as monotherapy or in combination with nucleoside reverse transcriptase inhibitors has been evaluated in 1446 patients enrolled in two double-blind, randomized trials.
14.1 Advanced Patients with Prior Antiretroviral Therapy
Study 247 was a randomized, double-blind trial (with open-label follow-up) conducted in HIV-infected patients with at least nine months of prior antiretroviral therapy and baseline CD4 cell counts less than or equal to 100 cells per μL. Ritonavir Tablets 600 mg twice-daily or placebo was added to each patient's baseline antiretroviral therapy regimen, which could have consisted of up to two approved antiretroviral agents. The study accrued 1,090 patients, with mean baseline CD4 cell count at study entry of 32 cells per μL. After the clinical benefit of ritonavir therapy was demonstrated, all patients were eligible to switch to open-label ritonavir for the duration of the follow-up period. Median duration of double-blind therapy with ritonavir and placebo was 6 months. The median duration of follow-up through the end of the open-label phase was 13.5 months for patients randomized to ritonavir and 14 months for patients randomized to placebo.
The cumulative incidence of clinical disease progression or death during the double-blind phase of Study 247 was 26% for patients initially randomized to ritonavir compared to 42% for patients initially randomized to placebo. This difference in rates was statistically significant.
Cumulative mortality through the end of the open-label follow-up phase for patients enrolled in Study 247 was 18% (99/543) for patients initially randomized to ritonavir compared to 26% (142/547) for patients initially randomized to placebo. This difference in rates was statistically significant. However, since the analysis at the end of the open-label phase includes patients in the placebo arm who were switched from placebo to ritonavir therapy, the survival benefit of ritonavir cannot be precisely estimated.
During the double-blind phase of Study 247, CD4 cell counts increases from baseline for patients randomized to ritonavir at Week 2 and Week 4 were observed. From Week 4 and through Week 24, mean CD4 cell counts for patients randomized to ritonavir appeared to plateau. In contrast, there was no apparent change in mean CD4 cell counts for patients randomized to placebo at any visit between baseline and Week 24 of the double-blind phase of Study 247.
14.2 Patients without Prior Antiretroviral Therapy
In Study 245, 356 antiretroviral-naive HIV-infected patients (mean baseline CD4 = 364 cells per μL) were randomized to receive either ritonavir tablets 600 mg twice-daily, zidovudine 200 mg three-times-daily, or a combination of these drugs.
During the double-blind phase of study 245, greater mean CD4 cell count increases were observed from baseline to Week 12 in the ritonavir-containing arms compared to the zidovudine arms. Mean CD4 cell count changes subsequently appeared to plateau through Week 24 in the ritonavir arm, whereas mean CD4 cell counts gradually diminished through Week 24 in the zidovudine and ritonavir plus zidovudine arms.
Greater mean reductions in plasma HIV-1 RNA levels were observed from baseline to Week 2 for the ritonavir-containing arms compared to the zidovudine arm. After Week 2 and through Week 24, mean plasma HIV-1 RNA levels either remained stable in the ritonavir and zidovudine arms or gradually rebounded toward baseline in the ritonavir plus zidovudine arm.
15 REFERENCES
- Sewester CS. Calculations. In: Drug Facts and Comparisons. St. Louis, MO: J.B. Lippincott Co; January, 1997:xix.
16 HOW SUPPLIED/STORAGE AND HANDLING
The package size, strength, and storage and handling recommendations for Ritonavir tablets, are shown in the table below.
| Ritonavir Tablets, 100 mg Ritonavir |
|
| Presentation | Ritonavir Tablets are white film-coated ovaloid tablets debossed with the "a" logo and the code NK. |
| Packaging Size | Bottles of 30 tablets each |
| NDC Number | 68382-696-06 |
| Recommended Storage | Store at or below 30°C (86°F). Exposure to temperatures up to 50°C (122°F) for seven days permitted. Dispense in original container or USP equivalent tight container (60 mL or less). For patient use: exposure of this product to high humidity outside the original or USP equivalent tight container (60 mL or less) for longer than 2 weeks is not recommended. |
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
General Administration Information [see Dosage and Administration (2)]:
- Advise patients and caregivers to pay special attention to accurate preparation and administration of their dose to minimize the risk of accidental overdose or underdose of ritonavir.
- Advise caregivers to inform their healthcare provider if their children’s weight changes in order to make sure that the child’s ritonavir dose is adjusted as needed.
- Advise patients to take ritonavir with meals.
- For adult patients taking ritonavir tablets, the maximum dose of 600 mg twice daily by mouth with meals should not be exceeded.
- Advise patients to remain under the care of a physician while using ritonavir and to take ritonavir and other concomitant antiretroviral therapy every day as prescribed. Ritonavir must always be used in combination with other antiretroviral drugs. Advise patients not to alter the dose or discontinue therapy without consulting with their healthcare provider. If a dose of ritonavir is missed patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped the patient should not double the next dose.
- Continued ritonavir therapy at a dose of 600 mg twice daily following loss of viral suppression may increase the likelihood of cross-resistance to other protease inhibitors.
- Ritonavir is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using ritonavir.
- Ritonavir may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John's Wort.
- Instruct patients receiving combined hormonal contraception to use an effective alternative contraceptive method or an additional barrier method during therapy with ritonavir because hormonal levels may decrease [see Drug Interactions (7.2), Use in Specific Populations (8.3)].
Pre-existing liver disease including Hepatitis B or C can worsen with use of ritonavir. This can be seen as worsening of transaminase elevations or hepatic decompensation. Advise patients that their liver function tests will need to be monitored closely especially during the first several months of ritonavir treatment and that they should notify their healthcare provider if they develop the signs and symptoms of worsening liver disease including loss of appetite, abdominal pain, jaundice, and itchy skin [see Warnings and Precautions (5.2)].
Pancreatitis, including some fatalities, has been observed in patients receiving ritonavir therapy. Advise patients to notify their healthcare provider of signs and symptoms (nausea, vomiting, and abdominal pain) that might be suggestive of pancreatitis [see Warnings and Precautions (5.3)].
Allergic Reactions/Hypersensitivity
Skin rashes ranging in severity from mild to Stevens-Johnson syndrome have been reported in patients receiving ritonavir. Advise patients to contact their healthcare provider if they develop a rash while taking ritonavir [see Warnings and Precautions (5.4)].
Ritonavir may produce changes in the electrocardiogram (e.g., PR prolongation). Advise patients to consult their healthcare provider if they experience symptoms such as dizziness, lightheadedness, abnormal heart rhythm or loss of consciousness [see Warnings and Precautions (5.5)].
Advise patients that treatment with ritonavir therapy can result in substantial increases in the concentration of total cholesterol and triglycerides [see Warnings and Precautions (5.6)].
Diabetes Mellitus/Hyperglycemia
Advise patients that new onset of diabetes or exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported and to notify their healthcare provider if they develop the signs and symptoms of diabetes mellitus including frequent urination, excessive thirst, extreme hunger or unusual weight loss and/or an increased blood sugar while on ritonavir as they may require a change in their diabetes treatment or new treatment [see Warnings and Precautions (5.7)].
Immune Reconstitution Syndrome
Advise patients that immune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including ritonavir [see Warnings and Precautions (5.8)].
Advise patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long term health effects of these conditions are not known at this time [see Warnings and Precautions (5.9)].
Advise patients with hemophilia that they may experience increased bleeding when treated with protease inhibitors such as ritonavir [see Warnings and Precautions (5.10)].
Inform patients that there is an antiretroviral pregnancy registry that monitors fetal outcomes of pregnant women exposed to ritonavir [see Use in Specific Populations (8.1)].
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
Ritonavir Tablets are manufactured by:
AbbVie Inc.
North Chicago, IL 60064 USA
Zydus Pharmaceuticals USA Inc.
© 2019 AbbVie Inc. All rights reserved.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: December 2019
| RITONAVIR
ritonavir tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Zydus Pharmaceuticals USA Inc (156861945) |
