SCRUB-IN SURGICAL SCRUB BRUSH/SPONGE- povidone-iodine solution
Scrub-In Surgical Scrub Brush/Sponge by
Drug Labeling and Warnings
Scrub-In Surgical Scrub Brush/Sponge by is a Otc medication manufactured, distributed, or labeled by Medline Industries, LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
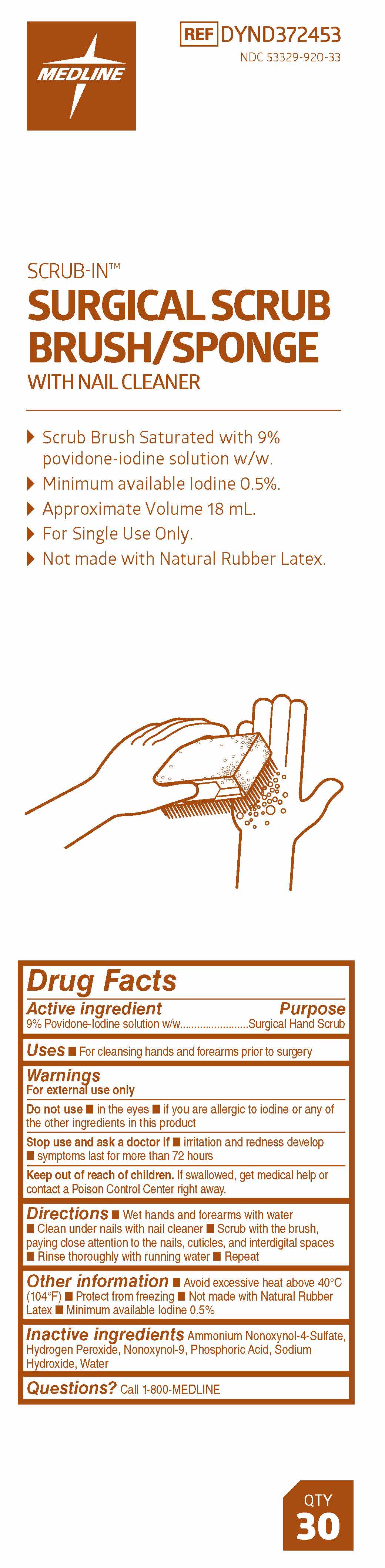
- Directions
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
REF DYND372453
NDC: 53329-920-33
Scrub-In TM
SURGICAL SCRUB BRUSH/SPONGE
WITH NAIL CLEANER
- Scrub Brush Saturated with 9% Povidone-Iodine solution w/w.
- Minimum available Iodine 0.5%.
- Approximate Volume 18 mL.
- For Single Use Only
- Not made with Natural Rubber Latex
QTY 30

-
INGREDIENTS AND APPEARANCE
SCRUB-IN SURGICAL SCRUB BRUSH/SPONGE
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53329-920 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 0.9 g in 100 mL Inactive Ingredients Ingredient Name Strength AMMONIUM NONOXYNOL-4 SULFATE (UNII: 9HIA70O4J0) HYDROGEN PEROXIDE (UNII: BBX060AN9V) NONOXYNOL-9 (UNII: 48Q180SH9T) PHOSPHORIC ACID (UNII: E4GA8884NN) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53329-920-33 30 in 1 BOX 11/12/2019 1 NDC: 53329-920-09 18 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 10/01/2012 Labeler - Medline Industries, Inc. (025460908) Establishment Name Address ID/FEI Business Operations Medline Industries, Inc. 185862984 manufacture(53329-920) , analysis(53329-920)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.