AKTEN- lidocaine hydrochloride anhydrous gel
AKTEN by
Drug Labeling and Warnings
AKTEN by is a Prescription medication manufactured, distributed, or labeled by Thea Pharma Inc., Akorn AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AKTEN ® safely and effectively. See full prescribing information for Akten ®.
AKTEN ® (lidocaine hydrochloride ophthalmic gel), for topical ophthalmic use.
Initial U.S. Approval: 1972RECENT MAJOR CHANGES
AKTEN is an amide local anesthetic indicated for ocular surface anesthesia during ophthalmologic
procedures. ( 1)RECENT MAJOR CHANGES
The recommended dose of AKTEN is 2 drops applied to the ocular surface in the area of the planned procedure. AKTEN may be reapplied to maintain anesthetic effect. ( 2)
RECENT MAJOR CHANGES
Ophthalmic Gel: 3.5% lidocaine hydrochloride in single-patient-use tubes. ( 3)
RECENT MAJOR CHANGES
- For Topical Ophthalmic Use: AKTEN is not for injection or intraocular administration. ( 5.1)
- Patients should not touch the eye for at least 10 to 20 minutes after using AKTEN as accidental injuries can occur due to insensitivity of the eye. ( 5.2)
- Corneal Opacification: Prolonged use of a topical ocular anesthetic may produce permanent corneal opacification and ulceration with accompanying visual loss. ( 5.2)
- For Administration by Healthcare Provider: AKTEN is not intended for patient self-administration. ( 5.3)
RECENT MAJOR CHANGES
Most common adverse reactions are conjunctival hyperemia, corneal epithelial changes, headache, and burning upon instillation. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Thea Pharma Inc. at 1-833-838-4028 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
CONTRAINDICATIONS
None. ( 4)
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 For Topical Ophthalmic Use
5.2 Corneal Injury Due to Insensitivity
5.3 Corneal Opacification
5.4 For Administration by Healthcare Provider
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 4 CONTRAINDICATIONS
-
8 USE IN SPECIFIC POPULATIONS
.
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with AKTEN in pregnant women to inform a drug-associated risk. In animal reproduction studies, subcutaneous administration of lidocaine to pregnant rats at doses >800-fold the human dose based on body surface area did not result in adverse developmental effects.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
Lidocaine is secreted in human milk. The clinical significance of this observation is unknown. Caution should be exercised when AKTEN is administered to a nursing woman.8.4 Pediatric Use
The safety and effectiveness of AKTEN for ocular surface anesthesia during ophthalmologic procedures have been established in pediatric patients. Use of AKTEN for this indication has been extrapolated from adequate and well-controlled studies in adults and studies in pediatric patients using different formulations of lidocaine.
-
10 OVERDOSAGE
Prolonged use of a topical ocular anesthetic may produce permanent corneal opacification and ulceration with accompanying visual loss.
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution. However, topical ocular application of AKTEN is not expected to result in systemic exposure.
-
11 DESCRIPTION
AKTEN (lidocaine hydrochloride ophthalmic gel) 3.5% contains lidocaine hydrochloride, an amide local anesthetic, as a sterile ophthalmic gel for topical ophthalmic use. AKTEN does not contain an anti-microbial preservative. Lidocaine hydrochloride is designated chemically as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl) monohydrochloride with a molecular formula of C 14H 22N 2O ∙ HCl and molecular weight of 270.8. The structural formula of the active ingredient is:
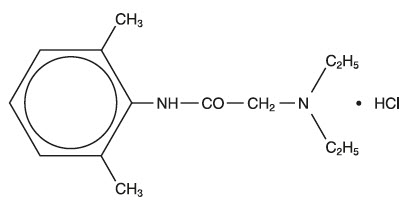
Each mL of AKTEN contains 35 mg of lidocaine hydrochloride as the active ingredient. Inactive ingredients: hypromellose, sodium chloride, and water for injection.
The pH may be adjusted to 5.5 to 7.5 with hydrochloric acid and/or sodium hydroxide. AKTEN does not contain an anti-microbial preservative. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lidocaine hydrochloride is an amide local anesthetic agent that stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action. Anesthesia generally occurs between 20 seconds to 1 minute and persists for 5 to 30 minutes.
12.3 Pharmacokinetics
Lidocaine may be absorbed following topical administration to mucous membranes. Its rate and extent of absorption depend upon various factors such as concentration, the specific site of application, viscosity of the agent, and duration of exposure.
The plasma binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein. Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N- dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacologic/toxicologic actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline.
Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged twofold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effect of AKTEN® on ocular anesthesia was studied in a multi-center, randomized, controlled, double-blind study. A total of 209 subjects were enrolled, with 54, 51, 53, and 51 subjects randomized to the sham, AKTEN® 1.5%, AKTEN® 2.5%, and AKTEN® 3.5% groups, respectively. Ocular anesthesia was achieved within 5 minutes of anesthetic application by 47 of 51 subjects (92%) in the AKTEN® 3.5% group.
The mean time to anesthesia onset ranged from 20 seconds to 5 minutes and was not affected by AKTEN® dose. The mean time to anesthesia onset was approximately 60 seconds, with a median onset time of 40 seconds for the AKTEN® 3.5% group. Among the subjects in the AKTEN® groups who achieved anesthesia within 5 minutes, approximately 90% had achieved anesthesia within 60 seconds of application.
The duration of anesthesia generally ranged from approximately 5 minutes to 30 minutes, with mean anesthesia durations of approximately 15 minutes for the AKTEN® 3.5% group.
Approximately 84% of the subjects in the AKTEN® 3.5% group experienced anesthesia for at least 5 minutes, approximately 55% of subjects experienced anesthesia for 10 minutes or longer and 27% experienced anesthesia for 15 minutes or longer. The anesthetic effect of additional applications of AKTEN® has not been evaluated.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
AKTEN (lidocaine hydrochloride ophthalmic gel) 3.5% is supplied as a clear gel in single-patient-use tubes as follows:
- * Made in Switzerland
NDC: 82584-792-01 1 mL fill in a white polyfoil tube * NDC: 82584-792-25 Package of 25 units of 1 mL fill in a white polyfoil tube * - 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
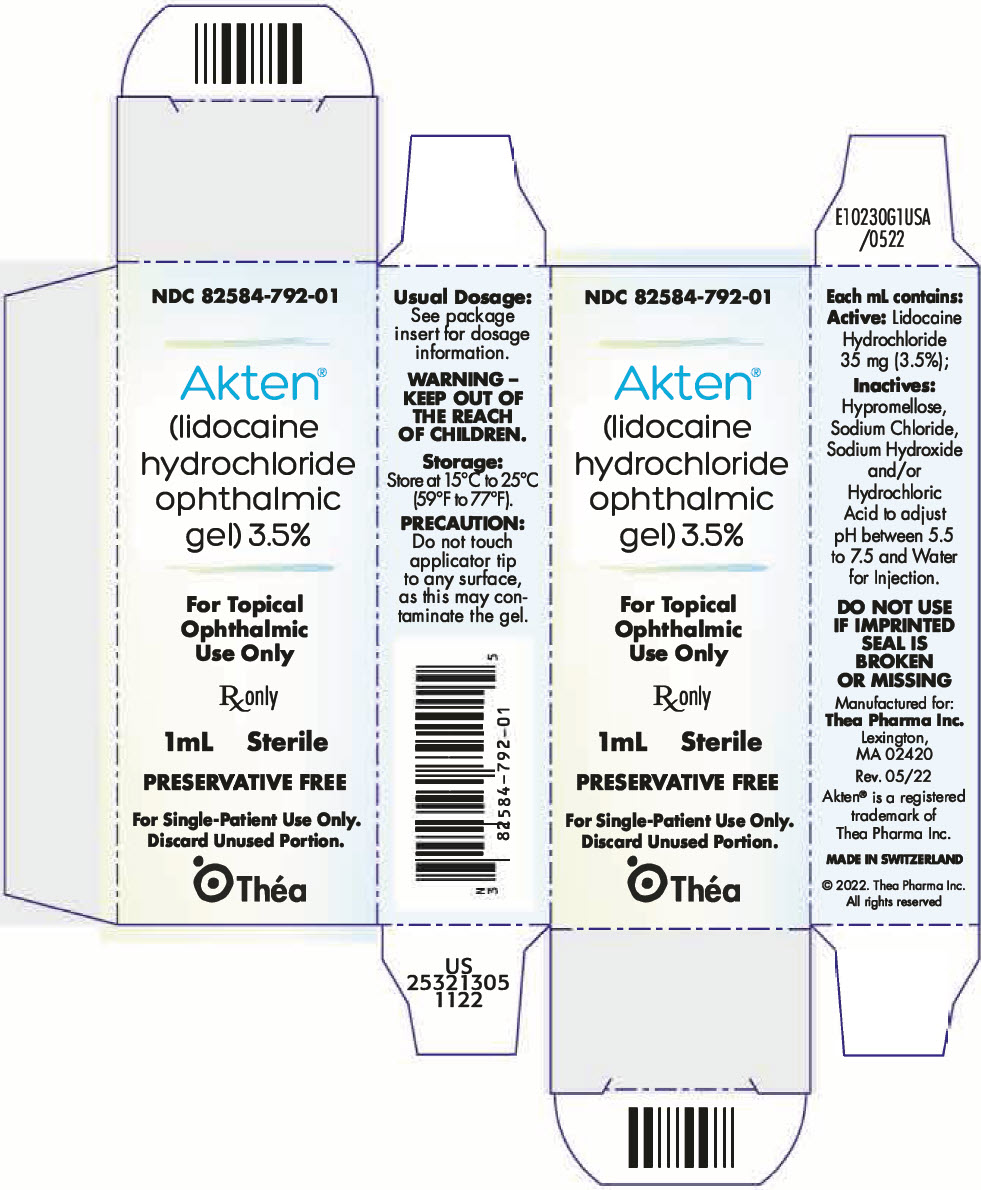
PRINCIPAL DISPLAY PANEL - 1 mL Tube Carton
NDC: 82584-792-01
Akten ®
(lidocaine
hydrochloride
ophthalmic
gel) 3.5%For Topical
Ophthalmic
Use OnlyRx only
1mL
SterilePRESERVATIVE FREE
For Single-Patient Use Only.
Discard Unused Portion.Théa
-
INGREDIENTS AND APPEARANCE
AKTEN
lidocaine hydrochloride anhydrous gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82584-792 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82584-792-01 1 in 1 CARTON 12/01/2022 1 1 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 82584-792-25 25 in 1 CARTON 12/01/2022 2 1 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022221 12/01/2022 Labeler - Thea Pharma Inc. (117787029)
Trademark Results [AKTEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AKTEN 77012922 3631872 Live/Registered |
Akorn Inc. 2006-10-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.