TYMLOS- abaloparatide injection, solution
Tymlos by
Drug Labeling and Warnings
Tymlos by is a Prescription medication manufactured, distributed, or labeled by Radius Health, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TYMLOS safely and effectively. See full prescribing information for TYMLOS.
TYMLOS ® (abaloparatide) injection, for subcutaneous use
Initial U.S. Approval: 2017WARNING: RISK OF OSTEOSARCOMA
See full prescribing information for complete boxed warning.
- Abaloparatide caused a dose-dependent increase in the incidence of osteosarcoma, a malignant bone tumor, in male and female rats. It is unknown whether TYMLOS will cause osteosarcoma in humans. ( 5.1, 13.1)
- Use of TYMLOS is not recommended in patients at increased risk for osteosarcoma. ( 5.1)
- Cumulative use of TYMLOS and parathyroid hormone analogs (e.g., teriparatide) for more than 2 years during a patient’s lifetime is not recommended. ( 5.1)
INDICATIONS AND USAGE
TYMLOS is a human parathyroid hormone related peptide [PTHrP(1-34)] analog indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture. ( 1)
DOSAGE AND ADMINISTRATION
- Recommended dose is 80 mcg subcutaneously once daily; patients should receive supplemental calcium and vitamin D if dietary intake is inadequate. ( 2.1)
- Administer as a subcutaneous injection into periumbilical region of abdomen. ( 2.2)
- Administer initially where the patient can sit or lie down in case symptoms of orthostatic hypotension occur. ( 2.2, 5.2)
DOSAGE FORMS AND STRENGTHS
Injection: 3120 mcg/1.56 mL (2000 mcg/mL) in a single-patient-use prefilled pen. The prefilled pen delivers 30 daily doses of 80 mcg abaloparatide in 40 mcL of sterile, clear, colorless solution. ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
- Orthostatic Hypotension: Instruct patients to sit or lie down if symptoms develop after dose administration. ( 5.2)
- Hypercalcemia: Avoid use in patients with pre-existing hypercalcemia and those known to have an underlying hypercalcemic disorder, such as primary hyperparathyroidism. ( 5.3)
- Hypercalciuria and Urolithiasis: Monitor urine calcium if preexisting hypercalciuria or active urolithiasis are suspected. ( 5.4)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) are hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain and vertigo. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Radius Health, Inc. at 1-855-672-3487 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF OSTEOSARCOMA
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Osteosarcoma
5.2 Orthostatic Hypotension
5.3 Hypercalcemia
5.4 Hypercalciuria and Urolithiasis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF OSTEOSARCOMA
- Abaloparatide caused a dose-dependent increase in the incidence of osteosarcoma (a malignant bone tumor) in male and female rats. The effect was observed at systemic exposures to abaloparatide ranging from 4 to 28 times the exposure in humans receiving the 80 mcg dose. It is unknown if TYMLOS will cause osteosarcoma in humans [see Warnings and Precautions ( 5.1) and Nonclinical Toxicology ( 13.1)] .
- The use of TYMLOS is not recommended in patients at increased risk of osteosarcoma including those with Paget's disease of bone or unexplained elevations of alkaline phosphatase, open epiphyses, bone metastases or skeletal malignancies, hereditary disorders predisposing to osteosarcoma, or prior external beam or implant radiation therapy involving the skeleton [see Warnings and Precautions ( 5.1)] .
- Cumulative use of TYMLOS and parathyroid hormone analogs (e.g., teriparatide) for more than 2 years during a patient’s lifetime is not recommended [see Warnings and Precautions ( 5.1)] .
-
1 INDICATIONS AND USAGE
TYMLOS is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture defined as a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. In postmenopausal women with osteoporosis, TYMLOS reduces the risk of vertebral fractures and nonvertebral fractures [see Clinical Studies ( 14)] .
Limitations of Use
Because of the unknown relevance of the rodent osteosarcoma findings to humans, cumulative use of TYMLOS and parathyroid hormone analogs (e.g., teriparatide) for more than 2 years during a patient’s lifetime is not recommended [see Warnings and Precautions ( 5.1)]. -
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- The recommended dosage of TYMLOS is 80 mcg subcutaneously once daily.
- Cumulative use of TYMLOS and parathyroid hormone analogs (e.g., teriparatide) for more than 2 years during a patient’s lifetime is not recommended [see Warnings and Precautions ( 5.1)].
- Patients should receive supplemental calcium and vitamin D if dietary intake is inadequate.
2.2 Administration Instructions
- Administer TYMLOS as a subcutaneous injection into the periumbilical region of the abdomen. Rotate the site of the injection every day and administer at approximately the same time every day. Do not administer intravenously or intramuscularly.
- Administer the first several doses where the patient can sit or lie down if necessary, in case symptoms of orthostatic hypotension occur [see Warnings and Precautions ( 5.2) and Adverse Reactions ( 6.1)].
- TYMLOS is a clear and colorless solution. Visually inspect TYMLOS for particulate matter and discoloration prior to administration. Do not use if solid particles appear or if the solution is cloudy or colored.
- Provide appropriate training and instruction to patients and caregivers on the proper use of the TYMLOS pen.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Osteosarcoma
Abaloparatide caused a dose-dependent increase in the incidence of osteosarcoma in male and female rats after subcutaneous administration at exposures 4 to 28 times the human exposure at the clinical dose of 80 mcg [see Nonclinical Toxicology ( 13.1)]. It is unknown whether TYMLOS will cause osteosarcoma in humans.
The use of TYMLOS is not recommended in patients at increased risk of osteosarcoma including those with Paget's disease of bone or unexplained elevations of alkaline phosphatase, open epiphyses, bone metastases or skeletal malignancies, hereditary disorders predisposing to osteosarcoma, or prior external beam or implant radiation therapy involving the skeleton.
Cumulative use of TYMLOS and parathyroid hormone analogs (e.g., teriparatide) for more than 2 years during a patient’s lifetime is not recommended.
5.2 Orthostatic Hypotension
Orthostatic hypotension may occur with TYMLOS, typically within 4 hours of injection. Associated symptoms may include dizziness, palpitations, tachycardia or nausea, and may resolve by having the patient lie down. For the first several doses, TYMLOS should be administered where the patient can sit or lie down if necessary [see Adverse Reactions ( 6.1)].
5.3 Hypercalcemia
TYMLOS may cause hypercalcemia. TYMLOS is not recommended in patients with pre-existing hypercalcemia or in patients who have an underlying hypercalcemic disorder, such as primary hyperparathyroidism, because of the possibility of exacerbating hypercalcemia [see Adverse Reactions ( 6.1)].
5.4 Hypercalciuria and Urolithiasis
TYMLOS may cause hypercalciuria. It is unknown whether TYMLOS may exacerbate urolithiasis in patients with active or a history of urolithiasis. If active urolithiasis or pre-existing hypercalciuria is suspected, measurement of urinary calcium excretion should be considered [see Adverse Reactions ( 6.1)].
-
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Orthostatic Hypotension [see Warnings and Precautions ( 5.2)]
- Hypercalcemia [see Warnings and Precautions ( 5.3)]
- Hypercalciuria and Urolithiasis [see Warnings and Precautions ( 5.4)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Postmenopausal Women with Osteoporosis
The safety of TYMLOS was evaluated in a randomized, multicenter, double-blind, placebo-controlled clinical trial in postmenopausal women with osteoporosis aged 49 to 86 years (mean age 69 years) who were randomized to receive 80 mcg of TYMLOS (N = 824) or placebo (N = 821), given subcutaneously once daily for 18 months [see Clinical Studies ( 14)] .In this study, the incidence of all-cause mortality was 0.4% in the TYMLOS group and 0.6% in the placebo group. The incidence of serious adverse events was 10% in the TYMLOS group and 11% in the placebo group. The percentage of patients who discontinued study drug due to adverse events was 10% in the TYMLOS group and 6% in the placebo group. The most common adverse reactions leading to study drug discontinuation in the TYMLOS group were nausea (2%), dizziness (1%), headache (1%), and palpitations (1%).
Table 1 shows the most common adverse reactions in the trial. These adverse reactions were generally not present at baseline, occurred more commonly with TYMLOS than with placebo, and occurred in at least 2% of the patients treated with TYMLOS.
Table 1: Common Adverse Reactions Reported in Postmenopausal Women with Osteoporosis* *Adverse reactions reported in ≥2% of TYMLOS-treated patients. Preferred term TYMLOS
(N=822)
(%)Placebo
(N=820)
(%)Hypercalciuria 11 9 Dizziness 10 6 Nausea 8 3 Headache 8 6 Palpitations 5 0.4 Fatigue 3 2 Abdominal pain upper 3 2 Vertigo 2 2 Orthostatic Hypotension
In the clinical trial of women with postmenopausal osteoporosis, the incidence of orthostatic blood pressure decline ≥20 mmHg systolic or ≥10 mmHg diastolic at 1 hour after the first injection was 4% in the TYMLOS group and 3% in the placebo group. At later time points the incidence was generally similar between the treatment groups. Adverse reactions of orthostatic hypotension were reported in 1% of patients receiving TYMLOS and 0.5% of patients receiving placebo. Dizziness was reported by more TYMLOS-treated patients (10%) compared to placebo (6%) [see Warnings and Precautions ( 5.2)].
Tachycardia
In women with postmenopausal osteoporosis, adverse reactions of tachycardia, including sinus tachycardia, were reported in 2% of patients receiving TYMLOS and 1% of patients in the placebo group. In 5 of the 13 patients receiving TYMLOS who experienced tachycardia, symptoms occurred within 1 hour of administration. TYMLOS has been associated with a dose-dependent increase in heart rate which developed within 15 minutes after injection and resolved in about 6 hours [see Clinical Pharmacology ( 12.2) ].
Injection Site Reactions
During the first month of the trial, injection site reactions were assessed daily one-hour after injection. TYMLOS had a higher incidence than placebo of injection site redness (58% vs. 28%), edema (10% vs. 3%) and pain (9% vs. 7%). Severe redness, severe edema, and severe pain were reported among 2.9%, 0.4%, and 0.4% of the TYMLOS-treated patients.Laboratory Abnormalities
Hypercalcemia
In the clinical trial of women with postmenopausal osteoporosis, TYMLOS caused increases in serum calcium concentrations [see Warnings and Precautions ( 5.3)] . The incidence of hypercalcemia, defined as albumin-corrected serum calcium ≥10.7 mg/dL at 4 hours following injection at any visit was 3% in TYMLOS-treated patients and 0.1% with placebo. Pre-dose serum calcium was similar to baseline in both groups. There were 2 (0.2%) TYMLOS-treated patients and no placebo-treated patients who discontinued from the study due to hypercalcemia. The incidence of hypercalcemia with TYMLOS was higher in patients with mild or moderate renal impairment (4%) compared to patients with normal renal function (1%).
Increases in Serum Uric Acid
TYMLOS increased serum uric acid concentrations. In the postmenopausal osteoporosis trial, among patients with normal baseline uric acid concentrations, 25% of patients in the TYMLOS group and 6% of patients in the placebo group had at least one post-baseline concentration above the normal range. The hyperuricemia observed in TYMLOS-treated patients was not associated with an increase in adverse reactions of gout or arthralgia over that observed with placebo.
Hypercalciuria and Urolithiasis
In the clinical trial of women with postmenopausal osteoporosis, the overall incidence of urine calcium:creatinine ratio >400 mg/g was higher with TYMLOS than with placebo (20% vs 15%, respectively). Urolithiases were reported in 2.1% of TYMLOS-treated patients and 1.7% of placebo-treated patients.
Adverse Reactions from the Extension Study in Postmenopausal Women with Osteoporosis
Following 18 months of treatment with TYMLOS or placebo, 1139 women transitioned to treatment with alendronate 70 mg administered orally once weekly. The incidence of adverse events occurring during alendronate treatment was similar in patients with prior placebo or TYMLOS therapy [see Clinical Studies ( 14)] .
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to TYMLOS in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Of the patients receiving TYMLOS for 18 months, 49% (300/610) developed anti-abaloparatide antibodies, of these, 68% (201/297) developed neutralizing antibodies to abaloparatide. Of the patients with anti-abaloparatide antibodies tested for cross-reactivity, 2.3% (7/298) developed cross-reactivity to PTHrP, 43% (3/7) developed neutralizing antibodies to PTHrP, and 0% (0/298) developed cross-reactive antibodies to PTH. Antibody formation did not appear to have any clinically significant impact on safety or efficacy endpoints, including bone mineral density (BMD) response, fracture reduction, immune-related hypersensitivity or allergic reactions, or other adverse events.
Most of the patients with anti-abaloparatide antibodies during treatment with TYMLOS, 85% (256/300), had follow-up antibody measurements six months after completion of TYMLOS therapy. Among these patients, 56% (143/256) remained antibody positive.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
TYMLOS is not indicated for use in females of reproductive potential. There are no human data with TYMLOS use in pregnant women to inform any drug associated risks. Animal reproduction studies with abaloparatide have not been conducted.8.2 Lactation
Risk Summary
TYMLOS is not indicated for use in females of reproductive potential. There is no information on the presence of abaloparatide in human milk, the effects on the breastfed infant, or the effects on milk production.8.4 Pediatric Use
Safety and effectiveness of TYMLOS have not been established in pediatric patients. TYMLOS is not recommended for use in pediatric patients with open epiphyses or hereditary disorders predisposing to osteosarcoma because of an increased baseline risk of osteosarcoma [see Warnings and Precautions ( 5.1)].
8.5 Geriatric Use
Of the total number of patients in the postmenopausal osteoporosis clinical studies of TYMLOS, 82% were age 65 years and over, and 19% were age 75 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dosage adjustment is required for patients with mild, moderate, or severe renal impairment. A study of a single dose of TYMLOS 80 mcg given subcutaneously was conducted in subjects with normal renal function or mild, moderate, or severe renal impairment. The maximal concentration (C max) and area under the concentration-time curve (AUC) of abaloparatide increased 1.4- and 2.1-fold, respectively, in subjects with severe renal impairment, compared to subjects with normal renal function. Patients with severe renal impairment may have increased abaloparatide exposure that may increase the risk of adverse reactions; therefore, monitor for adverse reactions [see Clinical Pharmacology ( 12.3)].
-
10 OVERDOSAGE
In a clinical study, accidental overdose was reported in a patient who received 400 mcg in one day (5 times the recommended clinical dose); dosing was temporarily interrupted. The patient experienced asthenia, headache, nausea, and vertigo. Serum calcium was not assessed on the day of the overdose, but on the following day the patient’s serum calcium was within the normal range. The effects of overdose may include hypercalcemia, nausea, vomiting, dizziness, tachycardia, orthostatic hypotension, and headache.
Overdosage Management
There is no specific antidote for TYMLOS. Treatment of suspected overdose should include discontinuation of TYMLOS, monitoring of serum calcium and phosphorus, and implementation of appropriate supportive measures, such as hydration. Based on the molecular weight, plasma protein binding and volume of distribution, abaloparatide is not expected to be dialyzable. -
11 DESCRIPTION
TYMLOS injection for subcutaneous administration contains abaloparatide, a synthetic 34 amino acid peptide. Abaloparatide is an analog of human parathyroid hormone related peptide, PTHrP(1-34). It has 41% homology to hPTH(1-34) (human parathyroid hormone 1-34) and 76% homology to hPTHrP(1-34) (human parathyroid hormone-related peptide 1-34).
Abaloparatide has a molecular formula of C 174 H 300 N 56 O 49 and a molecular weight of 3961 daltons with the amino acid sequence shown below:
- Ala-Val-Ser-Glu-His-Gln-Leu-Leu-His-Asp-Lys-Gly-Lys-Ser-Ile-Gln-Asp-Leu-Arg-Arg-Arg-Glu-Leu-Leu-Glu-Lys-Leu-Leu-Aib-Lys-Leu-His-Thr-Ala-NH 2
TYMLOS injection is supplied as a sterile, colorless, clear solution in a glass cartridge which is pre-assembled into a disposable single-patient-use pen. The pen is intended to deliver 30 once daily abaloparatide doses of 80 mcg in 40 mcL. Each cartridge contains 1.56 mL of TYMLOS solution. Each mL contains 2000 mcg abaloparatide and the following inactive ingredients: 5 mg phenol, 5.08 mg sodium acetate trihydrate, 6.38 mg acetic acid, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Abaloparatide is a PTHrP(1-34) analog which acts as an agonist at the PTH1 receptor (PTH1R). This results in activation of the cAMP signaling pathway in target cells. In rats and monkeys, abaloparatide had an anabolic effect on bone, demonstrated by increases in BMD and bone mineral content (BMC) that correlated with increases in bone strength at vertebral and/or nonvertebral sites [see Nonclinical Toxicology ( 13.2)].
12.2 Pharmacodynamics
Effects on Markers of Bone Turnover
A dose-finding study of abaloparatide administered once daily for 24 weeks demonstrated a dose-response relationship for BMD and bone formation markers.
Daily administration of TYMLOS to postmenopausal women with osteoporosis in clinical studies increased the bone formation marker serum procollagen type I N-propeptide (PINP). The increase in PINP levels peaked at Month 1 at 93% above baseline then decreased slowly over time. The increase in PINP was maintained above baseline throughout the treatment duration. At Month 18, PINP concentrations were approximately 45% above baseline. The increase in the bone resorption marker serum collagen type I cross-linked C-telopeptide (sCTX) peaked at Month 3 at 43% above baseline then decreased to 20% above baseline by Month 18.
Cardiac Electrophysiology
A 4-way cross-over thorough QT/QTc study was conducted in 55 healthy subjects who received single doses of placebo, subcutaneous doses of abaloparatide at 80 mcg and 240 mcg (three times the recommended dose), and moxifloxacin 400 mg orally. Abaloparatide increased heart rate, with a mean peak increase of 15 beats per minute (bpm) and 20 bpm at the first time point (15 minutes) after dosing with 80 mcg and 240 mcg, respectively. There were no clinically meaningful effects of abaloparatide on QTcI (individually corrected QT intervals) or cardiac electrophysiology.
12.3 Pharmacokinetics
Following seven days of subcutaneous administration of abaloparatide 80 mcg, the mean (SD) abaloparatide exposure was 812 (118) pg/mL for C max and 1622 (641) pg·hr/mL for AUC 0-24.
Figure 1 below shows the mean (SD) abaloparatide pharmacokinetic profile in postmenopausal women (N = 8) on Day 7.
Figure 1. Mean Abaloparatide Pharmacokinetic Profile in Postmenopausal Women on Day 7
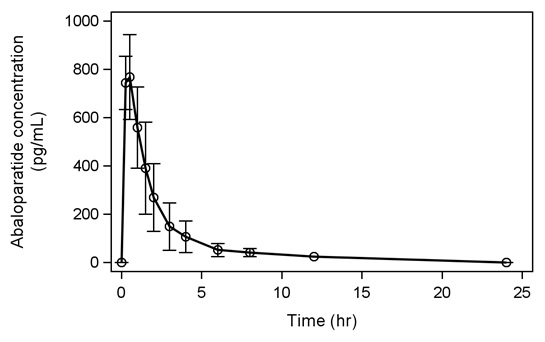
Absorption
The median (range) time to peak concentration of abaloparatide 80 mcg was 0.51 hr (0.25 to 0.52 hr) following subcutaneous administration. The absolute bioavailability of abaloparatide in healthy women after subcutaneous administration of an 80 mcg dose was 36%.
Distribution
The in vitro plasma protein binding of abaloparatide was approximately 70%. The volume of distribution was approximately 50 L.Elimination
The mean (SD) half-life of abaloparatide is 1.7 (0.7) hrs. The peptide fragments are primarily eliminated through renal excretion.Metabolism
No specific metabolism or excretion studies have been performed with TYMLOS. The metabolism of abaloparatide is consistent with non-specific proteolytic degradation into smaller peptide fragments, followed by elimination by renal clearance.Specific Populations
Geriatric Patients
No age-related differences in abaloparatide pharmacokinetics were observed in postmenopausal women ranging from 49 to 86 years of age.Race
No differences in abaloparatide pharmacokinetics based on race were observed in clinical trials.Patients with Renal Impairment
A single 80 mcg subcutaneous dose of abaloparatide was administered to male and female patients with renal impairment: 8 patients with mild renal impairment (CLCr 60 to 89 mL/min), 7 patients with moderate renal impairment (CLCr 30 to 59 mL/min), 8 patients with severe renal impairment (CLCr 15 to 29 mL/min), and 8 healthy subjects with normal renal function (CLCr 90 or greater mL/min) matched by sex, age, and body mass index (BMI). Abaloparatide C max increased 1.0-, 1.3-, and 1.4-fold in patients with mild, moderate, and severe renal impairment, compared to the healthy subjects with normal renal function. Abaloparatide AUC increased 1.2-, 1.7-, and 2.1-fold in patients with mild, moderate, and severe renal impairment, compared to the healthy subjects with normal renal function. Patients undergoing dialysis were not included in the study.Drug Interactions
In vitro studies showed that abaloparatide, at therapeutic concentrations, does not inhibit or induce Cytochrome P450 enzymes.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study, abaloparatide was administered once daily to male and female Fischer rats by subcutaneous injection at doses of 10, 25, and 50 mcg/kg. These doses resulted in systemic exposures to abaloparatide that were 4, 16, and 28 times, respectively, the systemic exposure observed in humans following the recommended subcutaneous dose of 80 mcg (based on AUC comparisons). Neoplastic changes related to treatment with abaloparatide consisted of marked dose-dependent increases in osteosarcoma and osteoblastoma incidence in all male and female dose groups. The incidence of osteosarcoma was 0-2% in untreated controls and reached 87% and 62% in male and female high-dose groups, respectively. The bone neoplasms were accompanied by marked increases in bone mass.
The relevance of the rat findings to humans is uncertain. The use of TYMLOS is not recommended in patients at increased risk of osteosarcoma [see Warnings and Precautions ( 5.1)].
Abaloparatide was not genotoxic or mutagenic in a standard battery of tests including the Ames test for bacterial mutagenesis, the chromosome aberration test using human peripheral lymphocytes, and the mouse micronucleus test.
13.2 Animal Toxicology and Pharmacology
In toxicity studies in rats and monkeys of up to 26-week and 39-week duration, respectively, findings included vasodilation, increases in serum calcium, decreases in serum phosphorus, and soft tissue mineralization at doses ≥10 mcg/kg/day. The 10 mcg/kg/day dose resulted in systemic exposures to abaloparatide in rats and monkeys that were 2 and 3 times, respectively, the exposure in humans at daily subcutaneous doses of 80 mcg.
Pharmacologic effects of abaloparatide on the skeleton were assessed in 12- and 16-month studies in ovariectomized (OVX) rats and monkeys, at doses up to 11 and 1 times human exposure at the recommended subcutaneous dose of 80 mcg, respectively (based on AUC comparisons). In these animal models of postmenopausal osteoporosis, treatment with abaloparatide resulted in dose-dependent increases in bone mass at vertebral and/or nonvertebral sites, correlating with increases in bone strength. The anabolic effect of abaloparatide was due to the predominant increase in osteoblastic bone formation and was evidenced by increases in trabecular thickness and/or cortical thickness due to endosteal bone apposition. Abaloparatide maintained or improved bone quality at all skeletal sites evaluated and did not cause any mineralization defects.
-
14 CLINICAL STUDIES
Efficacy Study in Women with Postmenopausal Osteoporosis
The efficacy of TYMLOS for the treatment of postmenopausal osteoporosis was evaluated in Study 003 (NCT 01343004), an 18-month, randomized, multicenter, double-blind, placebo-controlled clinical trial in postmenopausal women aged 49 to 86 years (mean age of 69) who were randomized to receive TYMLOS 80 mcg (N = 824) or placebo (N = 821) given subcutaneously once daily. Approximately 80% of patients were Caucasian, 16% were Asian, and 3% were Black; 24% were Hispanic. At baseline, the mean T-scores were -2.9 at the lumbar spine, -2.1 at the femoral neck, and -1.9 at the total hip. At baseline, 24% of patients had at least one prevalent vertebral fracture and 48% had at least one prior nonvertebral fracture. Patients took daily supplemental calcium (500 to 1000 mg) and vitamin D (400 to 800 IU).The efficacy study was extended as Study 005 (NCT 01657162), an open-label study where patients were no longer receiving TYMLOS or placebo but were maintained in their original randomized treatment group and received 70 mg alendronate weekly, with calcium and vitamin D supplements for 6 months. Study 005 enrolled 1139 patients, representing 92% of patients who completed Study 003. This included 558 patients who had previously received TYMLOS and 581 patients who had previously received placebo. The cumulative 25-month efficacy dataset included 18 months of exposure to TYMLOS or placebo in Study 003, 1 month of no treatment, followed by 6 months of alendronate therapy in Study 005. Study 005 was then continued to complete 18 months of additional alendronate exposure during which time patients were no longer blinded to their original Study 003 treatment group
Effect on New Vertebral Fractures
The primary endpoint was the incidence of new vertebral fractures in patients treated with TYMLOS compared to placebo. TYMLOS resulted in a significant reduction in the incidence of new vertebral fractures compared to placebo at 18 months (0.6% TYMLOS compared to 4.2% placebo, p ˂0.0001). The absolute risk reduction in new vertebral fractures was 3.6% at 18 months and the relative risk reduction was 86% for TYMLOS compared to placebo ( Table 2). The incidence of new vertebral fractures at 25 months was 0.6% in patients treated with TYMLOS then alendronate, compared to 4.4% in patients treated with placebo then alendronate (p ˂0.0001). The relative risk reduction in new vertebral fractures at 25 months was 87% for patients treated with TYMLOS then alendronate, compared to patients treated with placebo then alendronate, and the absolute risk reduction was 3.9% ( Table 2). After 24 months of open-label alendronate therapy, the vertebral fracture risk reduction achieved with TYMLOS therapy was maintained.
Table 2: Percentage of Postmenopausal Women with Osteoporosis with New Vertebral Fractures (modified Intent to Treat population)* † * Includes patients who had both pre- and post-treatment spine radiographs in Study 003
† Includes patients who had both pre- and post-treatment spine radiographs in Study 005
‡ Confidence IntervalPercentage of Postmenopausal
Women With FracturesAbsolute Risk
Reduction (%)
(95% CI‡)Relative Risk
Reduction (%)
(95% CI‡)TYMLOS
(N=690*)
(%)Placebo
(N=711*)
(%)0-18 months 0.6 4.2 3.6
(2.1, 5.4)86
(61, 95)TYMLOS/
Alendronate
(N=544 †)
(%)Placebo/
Alendronate
(N=568 †)
(%)0-25 months 0.6 4.4 3.9
(2.1, 5.9)87
(59, 96)Effect on Nonvertebral Fractures
TYMLOS resulted in a significant reduction in the incidence of nonvertebral fractures at the end of the 18 months of treatment plus 1 month follow-up where no drug was administered (2.7% for TYMLOS-treated patients compared to 4.7% for placebo-treated patients). The relative risk reduction in nonvertebral fractures for TYMLOS compared to placebo was 43% (logrank test p = 0.049) and the absolute risk reduction was 2.0%.
Following 6 months of alendronate treatment in Study 005, the cumulative incidence of nonvertebral fractures at 25 months was 2.7% for women in the prior TYMLOS group compared to 5.6% for women in the prior placebo group ( Figure 2). At 25 months, the relative risk reduction in nonvertebral fractures was 52% (logrank test p = 0.017) and the absolute risk reduction was 2.9%.
Figure 2. Cumulative Incidence of Nonvertebral Fractures* Over 25 Months (Intent to Treat Population) †
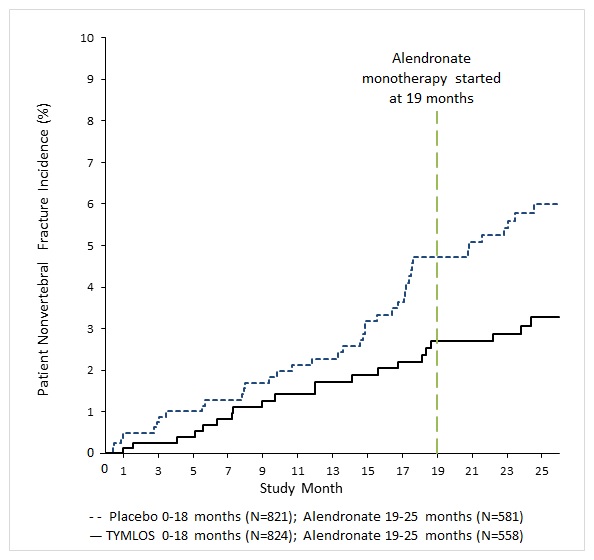
* Excludes fractures of the sternum, patella, toes, fingers, skull and face and those associated with high trauma.
† Includes patients randomized in Study 003
TYMLOS demonstrated consistent reductions in the risk of vertebral and nonvertebral fractures regardless of age, years since menopause, presence or absence of prior fracture (vertebral, nonvertebral) and BMD at baseline.
Effect on Bone Mineral Density (BMD)
Treatment with TYMLOS for 18 months in Study 003 resulted in significant increases in BMD compared to placebo at the lumbar spine, total hip and femoral neck, each with p<0.0001 ( Table 3). Similar findings were seen following 6 months of alendronate treatment in Study 005 ( Table 3).
Table 3: Mean Percent Changes in Bone Mineral Density (BMD) From Baseline to Endpoint in Postmenopausal Women with Osteoporosis (Intent to Treat Population) * † ‡ * Includes patients randomized in Study 003
† Includes patients enrolled in Study 005
‡Last-observation-carried-forward
§Confidence IntervalTYMLOS
(N=824 *)
(%)Placebo
(N=821 *)
(%)Treatment Difference (%)
(95% CI §)18 Months Lumbar Spine 9.2 0.5 8.8 (8.2, 9.3) Total Hip 3.4 -0.1 3.5 (3.3, 3.8) Femoral Neck 2.9 -0.4 3.3 (3.0, 3.7) TYMLOS/ Alendronate (N=558 †) (%) Placebo/ Alendronate (N=581 †) (%) 25 Months Lumbar Spine 12.8 3.5 9.3 (8.6, 10.1) Total Hip 5.5 1.4 4.1 (3.7, 4.5) Femoral Neck 4.5 0.5 4.1 (3.6, 4.6) TYMLOS demonstrated consistent increases in BMD regardless of age, years since menopause, race, ethnicity, geographic region, presence or absence of prior fracture (vertebral, nonvertebral), and BMD at baseline.
Effect on Bone Histology
Bone biopsy specimens were obtained from 71 patients with osteoporosis after 12 – 18 months of treatment (36 in the TYMLOS group and 35 in the placebo group). Of the biopsies obtained, 55 were adequate for quantitative histomorphometry assessment (27 in the TYMLOS group and 28 in the placebo group). Qualitative and quantitative histology assessment showed normal bone architecture and no evidence of woven bone, marrow fibrosis, or mineralization defects. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TYMLOS injection is supplied as a pre-assembled single-patient-use disposable pen (NDC: 70539-001-01) packaged in a cardboard carton (NDC: 70539-001-02) with the Instructions for Use and Medication Guide. Each disposable pen embodies a glass cartridge that contains 3120 mcg of abaloparatide in 1.56 mL (2000 mcg/mL) of sterilized, clear, colorless fluid. Each pen provides a 30-day supply for once daily injection of 80 mcg abaloparatide in 40 mcL.
Sterile needles are not included.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Medication Guide and Instructions for Use).
Risk of Osteosarcoma
Advise patients that the active ingredient in TYMLOS, abaloparatide, caused a dose-dependent increase in the incidence of osteosarcoma in male and female rats and that it is unknown whether TYMLOS will cause osteosarcoma in humans [see Warnings and Precautions ( 5.1)].Instruct patients to promptly report signs and symptoms of possible osteosarcoma such as persistent localized pain or occurrence of a new soft tissue mass that is tender to palpation.
Hypercalcemia
Advise patients that TYMLOS may cause hypercalcemia and discuss the symptoms of hypercalcemia (e.g., nausea, vomiting, constipation, lethargy, muscle weakness) [see Warnings and Precautions ( 5.3)].
Instruct patients to promptly report signs and symptoms of hypercalcemia.
Orthostatic Hypotension
Advise patients to sit or lie down if they feel lightheaded or have palpitations after the injection until their symptoms resolve. If these symptoms persist or worsen, advise patients to consult their healthcare provider before continuing treatment [see Dosage and Administration ( 2.2)].Use of TYMLOS Pen
Instruct patients and caregivers who administer TYMLOS on how to properly use the TYMLOS pen and to follow sharps disposal recommendations [see Dosage and Administration ( 2.2)]. Advise patients not to share their TYMLOS pen or needles with other patients and not to transfer the contents of the pen to a syringe.Advise patients that each TYMLOS pen can be used for up to 30 days, and after the 30-day use period, to discard the TYMLOS pen, even if it still contains unused solution [see How Supplied/Storage and Handling ( 16.2)] .
Manufactured in Germany for:
Radius Health, Inc.
950 Winter Street
Waltham, MA 02451TYMLOS is a registered trademark of Radius Health, Inc.
Copyright © 2017, Radius Health, Inc. All rights reserved.
Revised: October 2018
US-PI-000001-V2.0
-
MEDICATION GUIDE
MEDICATION GUIDE
TYMLOS ® (tim lows’)
(abaloparatide)
injection, for subcutaneous useWhat is the most important information I should know about TYMLOS?
TYMLOS may cause serious side effects including:-
Possible bone cancer (osteosarcoma). During animal drug testing, TYMLOS caused some rats to develop a bone cancer called osteosarcoma. It is not known if people who take TYMLOS will have a higher chance of getting osteosarcoma.
- Tell your healthcare provider right away if you have pain in your bones, pain in any areas of your body that does not go away, or any new or unusual lumps or swelling under your skin that is tender to touch.
What is TYMLOS? TYMLOS is a prescription medicine used to:
- decrease the chance of having a fracture of the spine and other bones in postmenopausal women with thinning and weakening bones (osteoporosis).
- treat osteoporosis in postmenopausal women who are at high risk for bone fracture.
It is not known if TYMLOS is safe and effective for children 18 years and younger.
It is not recommended that people use TYMLOS for more than 2 years during their lifetime.
TYMLOS should not be used in children and young adults whose bones are still growing.Before you take TYMLOS, tell your healthcare provider about all of your medical conditions, including if you: - have Paget’s disease of the bone or other bone disease.
- have or have had cancer in your bones.
- have or have had radiation therapy involving your bones.
- have or have had too much calcium in your blood.
- have or have had too much of an enzyme called alkaline phosphatase in your blood.
- have or have had an increase in your parathyroid hormone (hyperparathyroidism).
- will have trouble injecting yourself with the TYMLOS pen and do not have someone who can help you.
- are pregnant or plan to become pregnant. TYMLOS is not for pregnant women.
- are breastfeeding or plan to breastfeed. It is not known if TYMLOS passes into your breast milk. You and your healthcare provider should decide if you will take TYMLOS or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist each time you get a new medicine.How should I use TYMLOS? - Read the detailed Instructions for Use provided with your medicine.
- Use TYMLOS exactly as your healthcare provider tells you to use it.
- Do not try to inject TYMLOS yourself until you or your caregiver receive training from a healthcare provider on the right way to use the TYMLOS pen.
- You should receive your first several injections of TYMLOS where you can sit or lie down if necessary, until you know how it affects you.
- Inject TYMLOS 1 time each day into your lower stomach area (abdomen) just under your skin (subcutaneous). Avoid giving your injection within the 2-inch area around your belly button (navel).
- Talk to your healthcare provider about how to change (rotate) your injection site for each injection. Do not give TYMLOS in your veins (intravenously) or deep into your muscles (intramuscularly).
- You can take TYMLOS with or without food or drink.
- Take TYMLOS at about the same time each day.
- If you forget or cannot take TYMLOS at your usual time, take it as soon as you can on that day.
- The TYMLOS pen has enough medicine for 30 days. It is set to give 1 dose of medicine with each injection. Do not take more than 1 injection in the same day.
- Do not transfer the medicine from the TYMLOS pen to a syringe. This can cause you to use the wrong dose of TYMLOS. If you do not have pen needles to use with your TYMLOS pen, talk with your healthcare provider.
- Do not share your TYMLOS pen or pen needles with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- TYMLOS should look clear and colorless. Do not use TYMLOS if it has particles in it, or if it is cloudy or colored.
- Your healthcare provider may do blood and urine tests during your treatment with TYMLOS.
- Your healthcare provider may ask you to have a bone mineral density test after your treatment with TYMLOS.
- If you take more TYMLOS than prescribed you may experience symptoms such as muscle weakness, low energy, headache, nausea, dizziness (especially when getting up after sitting for a while) and a faster heartbeat. Stop taking TYMLOS and call your healthcare provider right away.
- If your healthcare provider recommends calcium and vitamin D supplements, you can take them while using TYMLOS.
What are the possible side effects of TYMLOS? TYMLOS can cause serious side effects including:
- See " What is the most important information I should know about TYMLOS?" at the end of this Medication Guide.
- Decrease in blood pressure when you change positions. Some people may feel dizzy, have a faster heartbeat, or feel lightheaded soon after the TYMLOS injection is given. These symptoms generally go away within a few hours. Take your injections of TYMLOS in a place where you can sit or lie down right away if you get these symptoms. If your symptoms get worse or do not go away, stop taking TYMLOS and call your healthcare provider.
- Increased blood calcium (hypercalcemia). TYMLOS can cause some people to have a higher blood calcium level than normal. Your healthcare provider may check your blood calcium before you start and during your treatment with TYMLOS. Tell your healthcare provider if you have nausea, vomiting, constipation, low energy, or muscle weakness. These may be signs there is too much calcium in your blood.
- Increased urine calcium (hypercalciuria). TYMLOS can cause some people to have higher levels of calcium in their urine than normal. Increased calcium may also cause you to develop kidney stones (urolithiasis) in your kidneys, bladder or urinary tract. Tell your healthcare provider right away if you get any symptoms of kidney stones which may include pain in your lower back or lower stomach area, pain when you urinate, or blood in your urine.
The most common side effects of TYMLOS include:
- dizziness
- nausea
- headache
- fast heartbeat
- feeling very tired (fatigue)
- upper stomach pain
- vertigo
These are not all the possible side effects of TYMLOS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TYMLOS? - Before first use, store TYMLOS pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- After first use, store your TYMLOS pen for up to 30 days at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze the TYMLOS pen or expose it to heat.
- Do not use TYMLOS after the expiration date printed on the pen and packaging.
- Throw away the TYMLOS pen after 30 days even if some medicine is left in the pen (see “ Instructions for Use” ).
Keep TYMLOS and all medicines out of the reach of children.
General information about the safe and effective use of TYMLOS.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TYMLOS for a condition for which it was not prescribed. Do not give TYMLOS to other people, even if they have the same condition you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about TYMLOS that is written for health professionals.What are the ingredients in TYMLOS?
Active ingredient: abaloparatide
Inactive ingredients: phenol, sodium acetate trihydrate, acetic acid, and water for injection.Radius
Radius Health, Inc., 950 Winter St., Waltham, MA 02451
Copyright © 2017, Radius Health, Inc. All rights reserved.
For more information about TYMLOS, visit our website at www.TYMLOS.com or call Radius Health, Inc., at 1-855-672-3487.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
TYMLOS is a registered trademark of Radius Health, Inc.
US-MG-000001-V2.0
Issued: 10/2018
-
Possible bone cancer (osteosarcoma). During animal drug testing, TYMLOS caused some rats to develop a bone cancer called osteosarcoma. It is not known if people who take TYMLOS will have a higher chance of getting osteosarcoma.
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
TYMLOS ® (tim lows’)
(abaloparatide)
injection, for subcutaneous use
Instructions for Use
Read and follow this Instructions for Use so that you inject TYMLOS pen the right way. Call your healthcare provider if you have any questions about the right way to inject the TYMLOS pen.
Important information about your TYMLOS pen
- Do not try to inject TYMLOS yourself until you or your caregiver receive training from a healthcare provider on the right way to use TYMLOS pen.
- Do not share your TYMLOS pen or pen needles with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- Each TYMLOS pen is a prefilled pen containing 30 doses of TYMLOS. Each dose will contain 80 mcg of TYMLOS. You do not have to measure your dose. The pen measures each dose of TYMLOS for you.
- Use a new pen needle for each injection. Keep the cap on the TYMLOS pen when not using the pen.
- Before using the pen always check the label to be sure it is your TYMLOS pen.
- Do not use your TYMLOS pen if it looks damaged. If you drop your TYMLOS pen or it becomes damaged, call your healthcare provider or pharmacist or call 1-855-672-3487.
- To clean your TYMLOS pen, wipe the outside of the pen with a clean, damp cloth, if needed.
- The TYMLOS pen is not recommended for use by the blind or visually impaired without the help of a person trained in the right way to use the pen.
Pen needles to use with your TYMLOS pen
- Pen needles are not included with your TYMLOS pen. You will need a prescription from your healthcare provider to get pen needles from your pharmacy.
- The correct needles to use with your TYMLOS pen are 5 to 8 mm, 31-gauge needles. Compatible needles include Clickfine®, BD Ultra-Fine™, MedtFine®, Easy Comfort, Clever Choice™ Comfort EZ™, and SureComfort™. If you are not sure what type of needle to use, ask your healthcare provider or pharmacist.
Supplies you will need for each injection using your TYMLOS pen
- 1 TYMLOS pen
- 1 pen needle
- 1 alcohol swab
- 1 cotton ball or gauze pad
- 1 sharps disposal container for pen needles and TYMLOS pens. See “ How should I throw away (dispose) of the pen needles and TYMLOS pens?” in the far right column.
How should I store the TYMLOS pen?
- Before first use, store TYMLOS pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not store pens with the needle attached.
- Do not freeze the TYMLOS pen or expose it to heat.
During 30 days of use:
- After first use, store the TYMLOS pen for up to 30 days at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the pen cap on your TYMLOS pen when storing. Do not store the TYMLOS pen with needle attached.
- Use your TYMLOS pen for only 30 days.
- Write down the date of your first use of TYMLOS here: _____/_____/_____. Throw away your TYMLOS pen 30 days after first opening it even if it still contains unused medicine.
Keep the TYMLOS pen, pen needles and all medicines out of the reach of children.
How should I throw away (dispose) of the pen needles and TYMLOS pens?
- Throw away (dispose of) your TYMLOS pen, in a FDA-cleared sharps disposal container or puncture resistant container, 30 days after first opening it even if it still contains unused medicine.
- Put your used pen needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) your used sharps disposal container in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes, and prefilled syringes.
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to FDA’s website at: https://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
For more information about the TYMLOS pen
For more information, go to www.TYMLOS.com or call 1-855-672-3487.
Manufactured in Germany.
Radius
Radius Health, Inc.
950 Winter Street
Waltham, MA 02451
TYMLOS is a registered trademark of Radius Health, Inc.
US-IFU-000001-V5.0
Begin the injection.
Check the TYMLOS ® pen
Step 1. Wash and dry your hands.
Step 2. Check the expiration date (Exp.) on the TYMLOS pen.
- Do not use the pen if the expiration date has passed.
- Call your healthcare provider or pharmacist if the expiration date has passed.
Attach pen needle to your TYMLOS pen
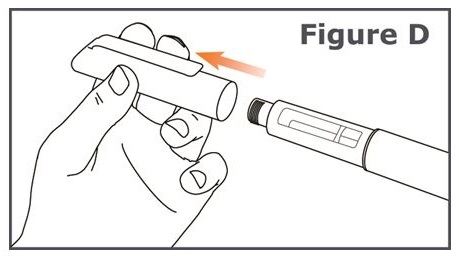
Step 3. Pull off the pen cap from your TYMLOS pen. (See Figure D.)
- Check the TYMLOS cartridge. The liquid should be clear, colorless, and free of particles; if not, do not use.
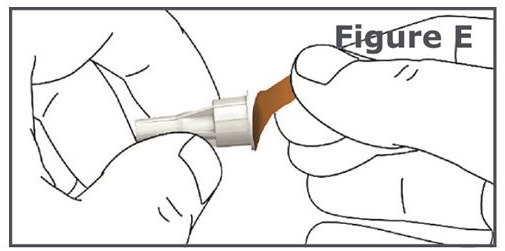
Step 4. Pull off the protective paper from the outer needle cap of your pen needle. (See Figure E.)
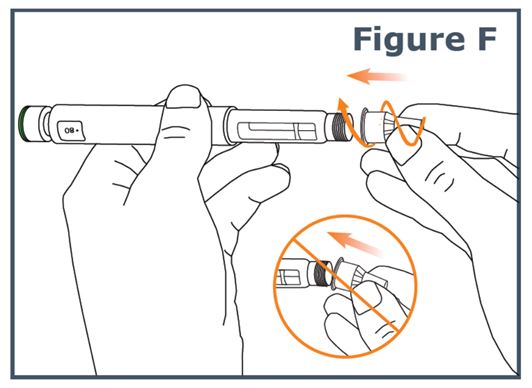
Step 5. Keep the needle straight and screw it onto the pen until fixed; a secure fit is ensured even if there is no noticeable stop. Do not over-tighten. (See Figure F.)
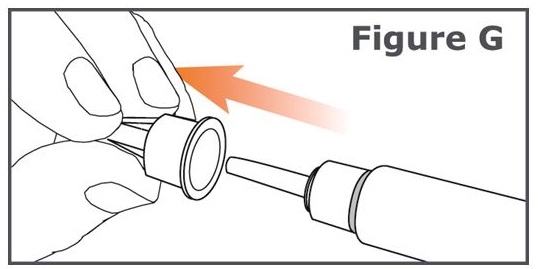
Step 6. Pull off the outer pen needle cap from the pen needle and keep it to use after your injection. (See Figure G.)
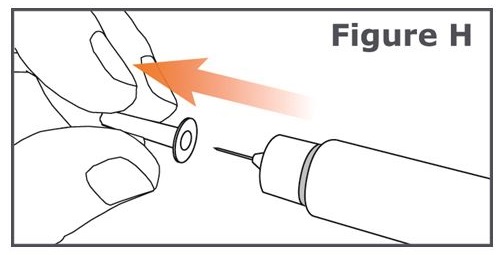
Step 7. Carefully pull off the inner pen needle cap and dispose of it. (See Figure H.)
Step 8. If you are using this pen for the first time, go to the “New pen setup - Day 1 Only” section to prime your pen.
- If you have already used this pen before, go to Step 13 for Days 2 through 30.
New pen setup – Day 1 Only (Priming your TYMLOS pen)

Do not repeat new pen setup on Days 2 through 30.
Step 9. The “New pen setup” removes air bubbles (primes your pen). You only need to prime your pen on Day 1 for each new pen. Otherwise, you will waste medicine.
- Skip Steps 10 through 12 on Days 2 through 30.
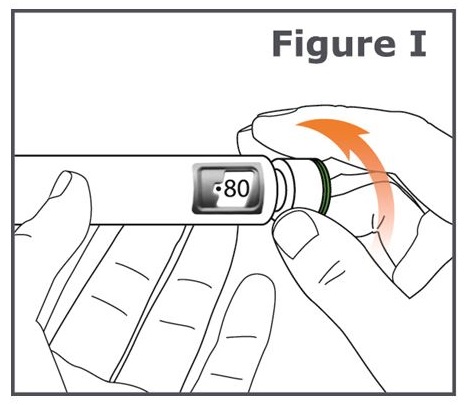
Step 10. Turn the dose knob on your TYMLOS pen away from you (clockwise) until it stops. (See Figure I.)
Note: You will see “ 80 ” lined up in the dose display window.
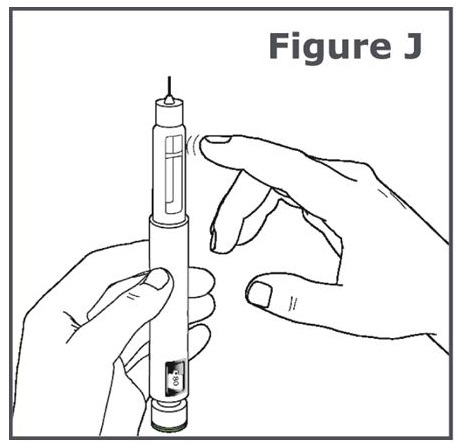
Step 11. Hold the TYMLOS pen with the pen needle pointing up. Tap lightly with your finger on the cartridge holder to move any air bubbles in the cartridge to the top of the cartridge. (See Figure J.)
Note: Do Step 11 even if you do not see air bubbles.
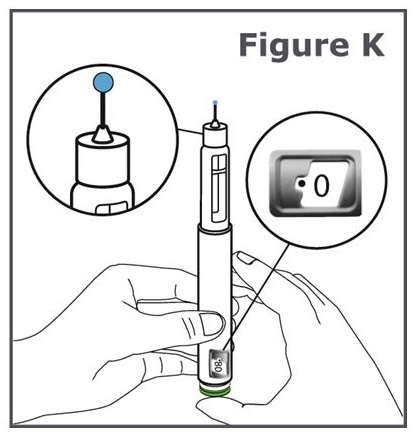
Step 12. Press the green injection button until it will not go any further. (See Figure K).
Note: You should see liquid come out of the needle tip.
Note: You will see “0” lined up in the dose display window.
What should I do if liquid does not come out of the needle tip?
- If you do not see a drop of TYMLOS, repeat Steps 10 through 12. A drop of liquid should come out of the needle tip.
- If you still do not see a drop of liquid call your healthcare provider or pharmacist and use a new TYMLOS pen.
- When you are ready to use your new TYMLOS pen for the first time, remember to repeat all Steps 9 through 12 to prime your new TYMLOS pen.
Set the dose on your TYMLOS pen
Do not push the green injection button while setting your dose.
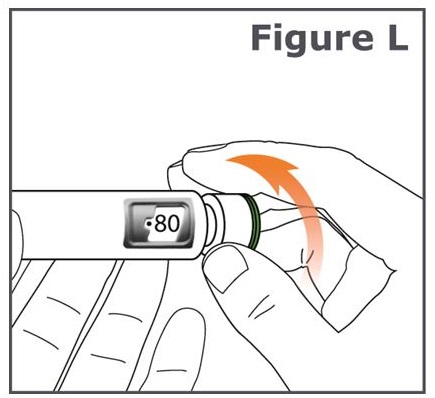
Step 13. Turn the dose knob on your pen away from you (clockwise) until the dose knob stops and “80” is lined up in the dose display window. (See Figure L.)
If you set the dose before you are ready to give your injection, turn the dose knob toward you (counter-clockwise) until “0” is in the dose display window.
If you are not able to set the TYMLOS pen to “80”, dispose of the pen and use a new TYMLOS pen for your injection, repeating Steps 1 through 13.
- If the pen cannot be set to “80”, there is not enough medicine in the TYMLOS pen for your full dose. See “ How should I throw away (dispose) of the pen needles and TYMLOS pens?” on the other side.
Choose and clean your injection site
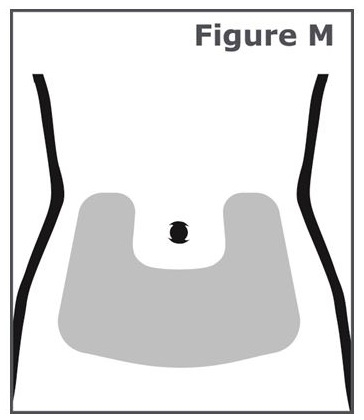
Step 14. Injections should be given in the lower stomach area (abdomen). (See Figure M.) Avoid the 2-inch area around your belly button (navel).
For each injection, change (rotate) your injection site around your abdomen. Talk to your healthcare provider about how to change (rotate) your injection site for each injection. Do not use the same injection area for each injection. Do not inject into areas where the skin is tender, bruised, red, scaly, or hard. Avoid areas with scars or stretch marks.
Step 15. Wipe the injection site with an alcohol swab and allow it to dry. Do not touch, fan, or blow on the injection site after you have cleaned it.
Giving your TYMLOS pen injection
Read Step 16 and Step 17 before you give your injection.
Inject your TYMLOS pen the way your healthcare provider has shown you.
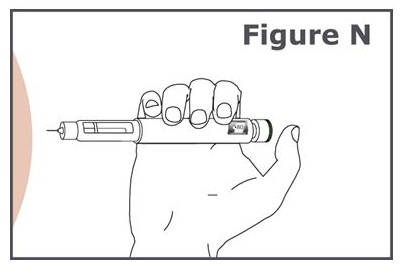
Step 16. (See Figure N.)
- Hold the pen so you can see the dose display window during the injection.
- Insert the pen needle straight into your skin.
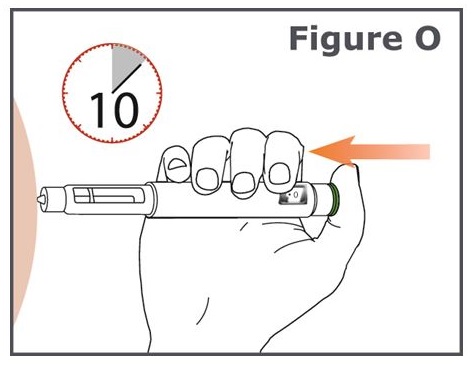
Step 17. (See Figure O.)
- Press the green injection button until it cannot go any further and “0” is in the dose display window. Do not move the TYMLOS pen after inserting the needle.
- Continue to press the green injection button while counting to 10. Counting to 10 will allow the full dose of TYMLOS to be given.
Step 18. After counting to 10, release your finger from the green injection button and slowly remove the TYMLOS pen from the injection site by pulling the pen needle straight out.
Remove the pen needle
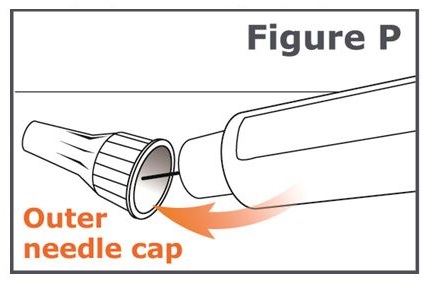
Step 19. Carefully place the outer needle cap back on the pen needle. Press on the outer needle cap until it snaps into place and is secure. (See Figure P.)
Caution: To prevent needle stick injury, carefully follow Step 20.
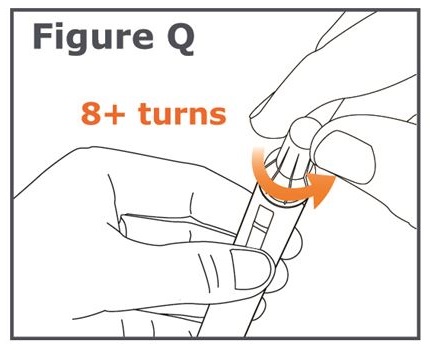
Step 20. Unscrew the capped needle (like unscrewing a cap from a bottle). To unscrew the capped needle you may need to turn it 8 or more turns and then gently pull until the capped needle comes off. (See Figure Q.)
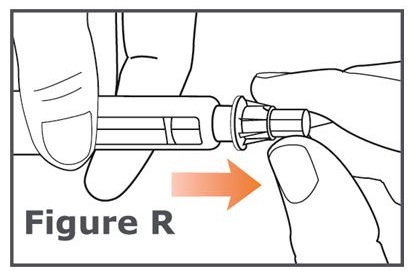
Note: Do not push on the outer needle cap while unscrewing the needle. You should see a gap widening between the outer needle cap and the pen as you unscrew the needle. (See Figure R.)
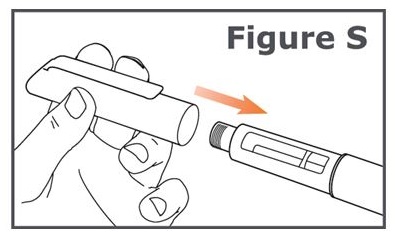
Replace pen cap
Step 21. Firmly replace the pen cap onto the TYMLOS pen. (See Figure S.)
Keep the pen cap on your TYMLOS pen between injections.After your injection
Step 22. Press a cotton ball or gauze pad over the injection site and hold for 10 seconds. Do not rub the injection site. You may have slight bleeding. This is normal.
You may cover the injection site with a small adhesive bandage, if needed.- Dispose of the pen needle. See “ How should I throw away (dispose) of the pen needles and TYMLOS pens?” .
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 10/2018
- PRINCIPAL DISPLAY PANEL - NDC: 70539-001-01 - 80 mcg Prefilled Pen Label
- PRINCIPAL DISPLAY PANEL - NDC: 70539-001-02 - 80 mcg Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 70539-001-99 - Sample 80 mcg Prefilled Pen Label
- PRINCIPAL DISPLAY PANEL - NDC: 70539-001-98 - Sample 80 mcg Carton Label
-
INGREDIENTS AND APPEARANCE
TYMLOS
abaloparatide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70539-001 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ABALOPARATIDE (UNII: AVK0I6HY2U) (ABALOPARATIDE - UNII:AVK0I6HY2U) ABALOPARATIDE 2000 ug in 1 mL Inactive Ingredients Ingredient Name Strength PHENOL (UNII: 339NCG44TV) 5 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 5.08 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) 6.38 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70539-001-02 1 in 1 BOX, UNIT-DOSE 05/01/2017 1 NDC: 70539-001-01 1.56 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 70539-001-98 1 in 1 BOX, UNIT-DOSE 05/01/2017 2 NDC: 70539-001-99 1.56 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208743 05/01/2017 Labeler - Radius Health, Inc. (146676262)
Trademark Results [Tymlos]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TYMLOS 97851998 not registered Live/Pending |
Radius Health, Inc. 2023-03-22 |
 TYMLOS 88640220 not registered Live/Pending |
Radius Health, Inc. 2019-10-03 |
 TYMLOS 86983687 5433991 Live/Registered |
Radius Health, Inc. 2016-03-25 |
 TYMLOS 86953480 not registered Dead/Abandoned |
Radius Health, Inc. 2016-03-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.