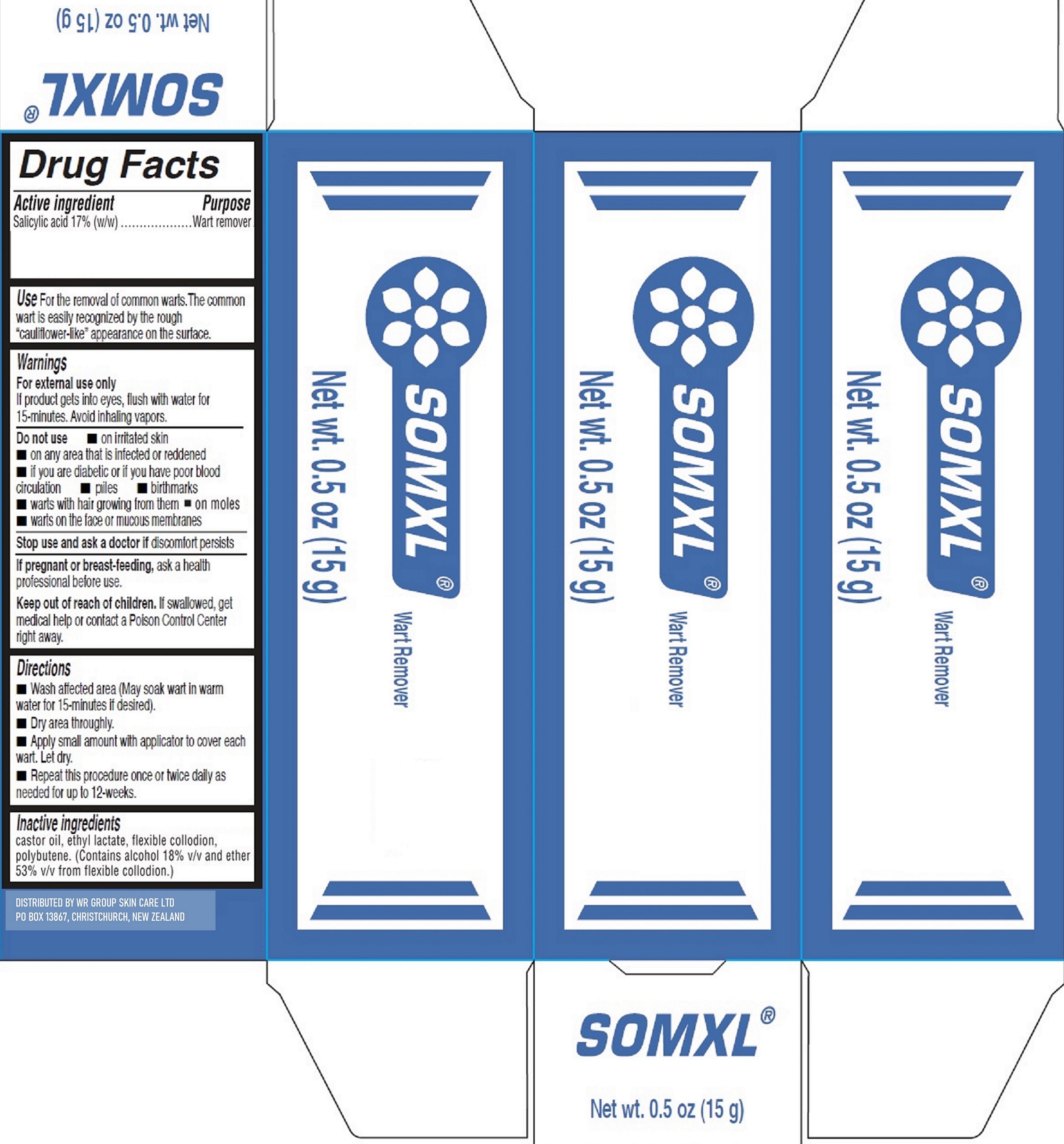
SOMXL by WR Group Skin Care Ltd
SOMXL by
Drug Labeling and Warnings
SOMXL by is a Otc medication manufactured, distributed, or labeled by WR Group Skin Care Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SOMXL- salicylic acid solution
WR Group Skin Care Ltd
----------
Use
For the removal of common warts. The common wart is easily recognized by the rough
"cauliflower-like" appearace on the surface.
Warnings
For external use only
If product gets into eyes, flush with water for 15-minutes. Avoid inhaling vapors.
Directions
- Wash affected area (May soak wart in warm water for 15-minutes if desired).
- Dry area throughly.
- Apply small amount with applicator to cover each wart. Let dry.
- Repeat this procedure once ot twice daily as needed for up to 12-weeks.
| SOMXL
salicylic acid solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - WR Group Skin Care Ltd (592719822) |
Revised: 8/2024
Document Id: 1ef652bd-6c4b-846e-e063-6394a90a9fd9
Set id: 7122eab1-c56b-440c-b853-62ae9dfede97
Version: 5
Effective Time: 20240805
Trademark Results [SOMXL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SOMXL 88263531 not registered Live/Pending |
McKinnon, Blair David 2019-01-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.