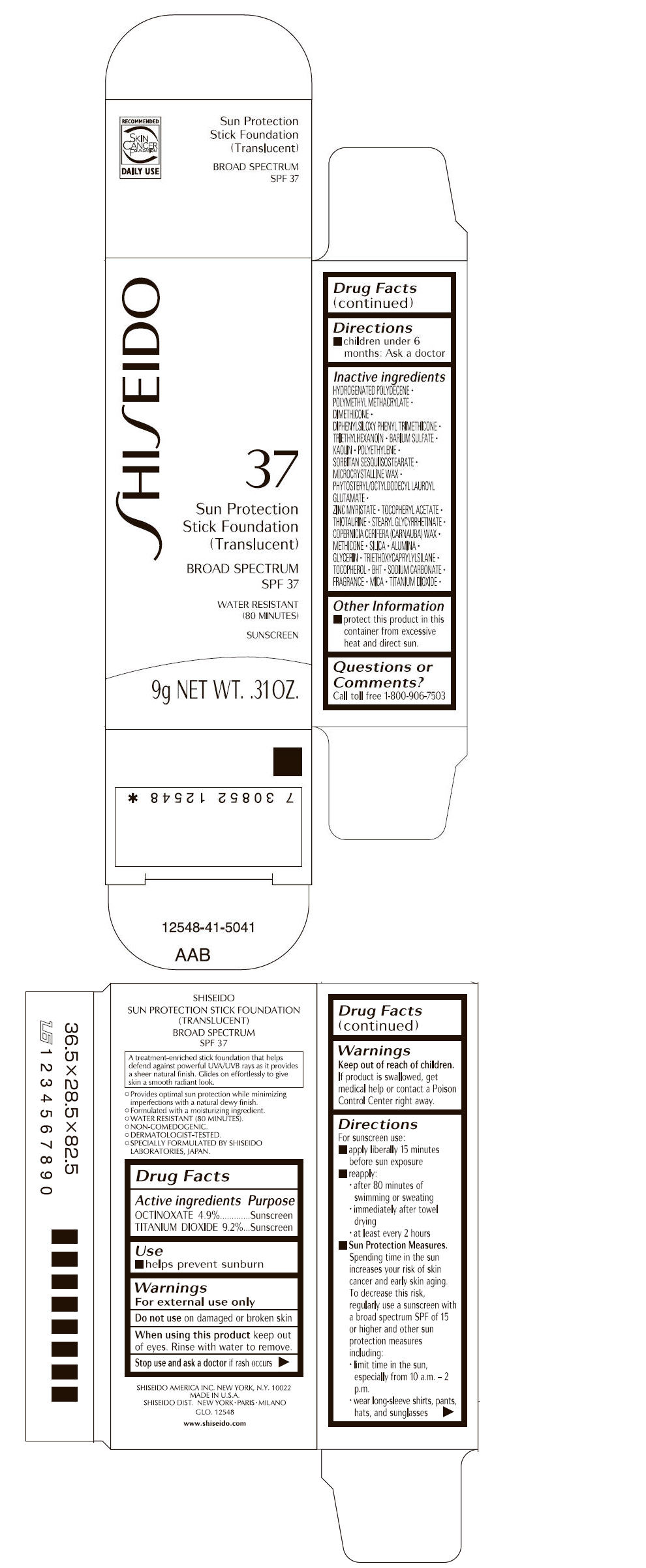
SHISEIDO SUN PROTECTION FOUNDATION TRANSLUCENT- octinoxate and titanium dioxide stick
SHISEIDO SUN PROTECTION FOUNDATION by
Drug Labeling and Warnings
SHISEIDO SUN PROTECTION FOUNDATION by is a Otc medication manufactured, distributed, or labeled by SHISEIDO AMERICA INC., Davlyn Industries Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
HYDROGENATED POLYDECENE, POLYMETHYL METHACRYLATE, DIMETHICONE, DIPHENYLSILOXY PHENYL TRIMETHICONE, TRIETHYLHEXANOIN, BARIUM SULFATE, KAOLIN, POLYETHYLENE, SORBITAN SESQUIISOSTEARATE, MICROCRYSTALLINE WAX, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, ZINC MYRISTATE, TOCOPHERYL ACETATE, THIOTAURINE, STEARYL GLYCYRRHETINATE, COPERNICIA CERIFERA (CARNAUBA) WAX, METHICONE, SILICA, ALUMINA, GLYCERIN, TRIETHOXYCAPRYLYLSILANE, TOCOPHEROL, BHT, SODIUM CARBONATE, FRAGRANCE, MICA, TITANIUM DIOXIDE,
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 9 g Cartridge Carton
-
INGREDIENTS AND APPEARANCE
SHISEIDO SUN PROTECTION FOUNDATION TRANSLUCENT
octinoxate and titanium dioxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52686-338 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 441 mg in 9 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 828 mg in 9 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) DIMETHICONE (UNII: 92RU3N3Y1O) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) BARIUM SULFATE (UNII: 25BB7EKE2E) KAOLIN (UNII: 24H4NWX5CO) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) ZINC MYRISTATE (UNII: K09A9E2GGO) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) THIOTAURINE (UNII: NQZ2D7AO62) STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) CARNAUBA WAX (UNII: R12CBM0EIZ) METHICONE (20 CST) (UNII: 6777U11MKT) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERIN (UNII: PDC6A3C0OX) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SODIUM CARBONATE (UNII: 45P3261C7T) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52686-338-20 1 in 1 CARTON 1 9 g in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2013 Labeler - SHISEIDO AMERICA INC. (782677132) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 MANUFACTURE(52686-338) , ANALYSIS(52686-338) Establishment Name Address ID/FEI Business Operations Davlyn Industries Inc 624436254 MANUFACTURE(52686-338)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.