ALBON- sulfadimethoxine tablet
Albon by
Drug Labeling and Warnings
Albon by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION
-
Description
Albon is a low-dosage, rapidly absorbed, long-acting sulfonamide, effective for the treatment of a wide range of bacterial infections commonly encountered in dogs and cats.
Sulfadimethoxine is a white, almost tasteless and odorless compound. Chemically, it is N1-(2,6-dimethoxy-4-pyrimidinyl) sulfanilamide. The structural formula is:
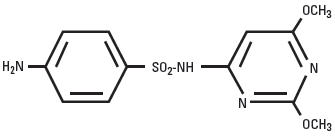
Action
Sulfadimethoxine has been demonstrated clinically or in the laboratory to be effective against a variety of organisms, such as streptococci, klebsiella, proteus, shigella, staphylococci, escherichia, and salmonella.1,2 These organisms have been demonstrated in respiratory, genitourinary, enteric, and soft tissue infections of dogs and cats.
The systemic sulfonamides which include sulfadimethoxine are bacteriostatic agents. Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from para-aminobenzoic acid. Mammalian cells are capable of utilizing folic acid in the presence of sulfonamides.
The tissue distribution of sulfadimethoxine, as with all sulfonamides, is a function of plasma levels, degree of plasma protein binding, and subsequent passive distribution in the tissues of the lipid-soluble un-ionized form. The relative amounts are determined by both its pKa and by the pH of each tissue. Therefore, levels tend to be higher in less acid tissue and body fluids or those diseased tissues having high concentrations of leucocytes.2
In the dog, sulfadimethoxine is not acetylated as in most other animals, and it is excreted predominantly as the unchanged drug.3 Sulfadimethoxine has a relatively high solubility at the pH normally occurring in the kidney, precluding the possibility of precipitation and crystalluria. Slow renal excretion results from a high degree of tubular reabsorption,4 and plasma protein binding is very high, providing a blood reservoir of the drug. Thus, sulfadimethoxine maintains higher blood levels than most other long-acting sulfonamides. Single, comparatively low doses of Albon give rapid and sustained therapeutic blood levels.1
To assure successful sulfonamide therapy (1) the drug must be given early in the course of the disease, and it must produce a high sulfonamide level in the body rapidly after administration, (2) therapeutically effective sulfonamide levels must be maintained in the body throughout the treatment period, (3) treatment should continue for a short period of time after the clinical signs have disappeared, and (4) the causative organisms must be sensitive to this class of drugs.
-
INDICATIONS AND USAGE
Albon is indicated for the treatment of respiratory, genitourinary tract, enteric, and soft tissue infections in dogs and cats:
tonsillitis
pharyngitis
bronchitis
pneumonia
cystitis
nephritis
metritis
pyometrapustular dermatitis
anal gland infections
abscesses
wound infections
bacterial enteritis
canine salmonellosis
bacterial enteritis associated
with coccidiosis in dogswhen caused by streptococci, staphylococci, escherichia, salmonella, klebsiella, proteus or shigella organisms sensitive to sulfadimethoxine.
Limitations: Sulfadimethoxine is not effective in viral or rickettsial infections, and as with any antibacterial agent, occasional failures in therapy may occur due to resistant microorganisms. The usual precautions in sulfonamide therapy should be observed.
- WARNING
- PRECAUTION
-
DOSAGE AND ADMINISTRATION
Initial Dose: 25 mg/lb (55 mg/kg) of animal body weight.
Subsequent Daily Doses: 12.5 mg/lb (27.5 mg/kg) of animal body weight.
For ease of administration in animals of varying weights, 3 tablet sizes are provided. The following table indicates how dosage may be adjusted depending on tablet size and body weight. Subsequent doses should be given at 24-hour intervals.
Tablet Size Approximate Animal Weight Initial Dose 25 mg/lb (55 mg/kg) Subsequent Daily Doses 12.5 mg/lb (27.5 mg/kg) 125 mg 5 lb (2.2 kg) 1 tablet 1/2 tablet 250 mg 10 lb (4.5 kg) 1 tablet 1/2 tablet 500 mg 20 lb (9.1 kg) 1 tablet 1/2 tablet Treatment may be initiated with Albon Injection 40% to obtain effective blood levels almost immediately or to facilitate treatment of the fractious animal.
Length of treatment depends on the clinical response. In most cases treatment for 3–5 days is adequate. Treatment should be continued until the animal is asymptomatic for 48 hours.
-
TOXICITY AND SAFETY
Data regarding acute and chronic toxicities of sulfadimethoxine indicate the drug is very safe. The LD50 in mice is greater than 2 g/kg of body weight when administered intraperitoneally and greater than 16 g/kg when administered orally. In dogs receiving massive single oral doses of 3.2 g/kg of body weight, diarrhea was the only adverse effect observed. Dogs given 160 mg/kg of body weight orally daily for 13 weeks showed no signs of toxicity.
- STORAGE
- HOW SUPPLIED
-
REFERENCES
1. Data on file, Pfizer Animal Health.
2. Stowe CM: The sulfonamides. In Jones LM (ed), Veterinary Pharmacology and Therapeutics, Ames, Iowa, Iowa State University Press, chapter 33, 1965.
3. Bridges JW, Kirby MR, Walker SR, et al: Species differences in the metabolism of sulfadimethoxine. Biochem J 109:851, 1968.
4. Baggot JD: Some aspects of drug persistence in domestic animals. Res Vet Sci 11(2):130, 1970.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 125 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ALBON
sulfadimethoxine tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8431 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFADIMETHOXINE (UNII: 30CPC5LDEX) (SULFADIMETHOXINE - UNII:30CPC5LDEX) SULFADIMETHOXINE 125 mg Product Characteristics Color WHITE Score 2 pieces Shape ROUND (Bi-convex, cylindrical) Size 8mm Flavor Imprint Code ALBON;125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8431-1 200 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA015102 12/14/1964 ALBON
sulfadimethoxine tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8432 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFADIMETHOXINE (UNII: 30CPC5LDEX) (SULFADIMETHOXINE - UNII:30CPC5LDEX) SULFADIMETHOXINE 250 mg Product Characteristics Color WHITE Score 2 pieces Shape ROUND (Flat faced, cylindrical with beveled edges) Size 10mm Flavor Imprint Code ALBON;250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8432-1 500 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA015102 12/14/1964 ALBON
sulfadimethoxine tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8433 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFADIMETHOXINE (UNII: 30CPC5LDEX) (SULFADIMETHOXINE - UNII:30CPC5LDEX) SULFADIMETHOXINE 500 mg Product Characteristics Color WHITE Score 4 pieces Shape ROUND (Flat faced, cylindrical with beveled edges) Size 13mm Flavor Imprint Code ALBON;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8433-1 100 in 1 BOTTLE, PLASTIC 2 NDC: 54771-8433-2 500 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA015102 12/14/1964 Labeler - Zoetis Inc. (828851555)
Trademark Results [Albon]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALBON 86397160 4802447 Live/Registered |
ZOETIS SERVICES LLC 2014-09-17 |
 ALBON 72160708 0760908 Dead/Cancelled |
Hoffmann-La Roche Inc. 1963-01-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


