Hello Unicorn by Hello Products LLC Hello® Unicorn
Hello Unicorn by
Drug Labeling and Warnings
Hello Unicorn by is a Otc medication manufactured, distributed, or labeled by Hello Products LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HELLO UNICORN- sodium fluoride gel, dentifrice
Hello Products LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hello® Unicorn
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
sorbitol, purified water, hydrated silica, glycerin, xylitol, xanthan gum, flavor, cocamidopropyl betaine, sodium cocoyl glutamate, stevia rebaudiana leaf extract, mica and titanium dioxide.
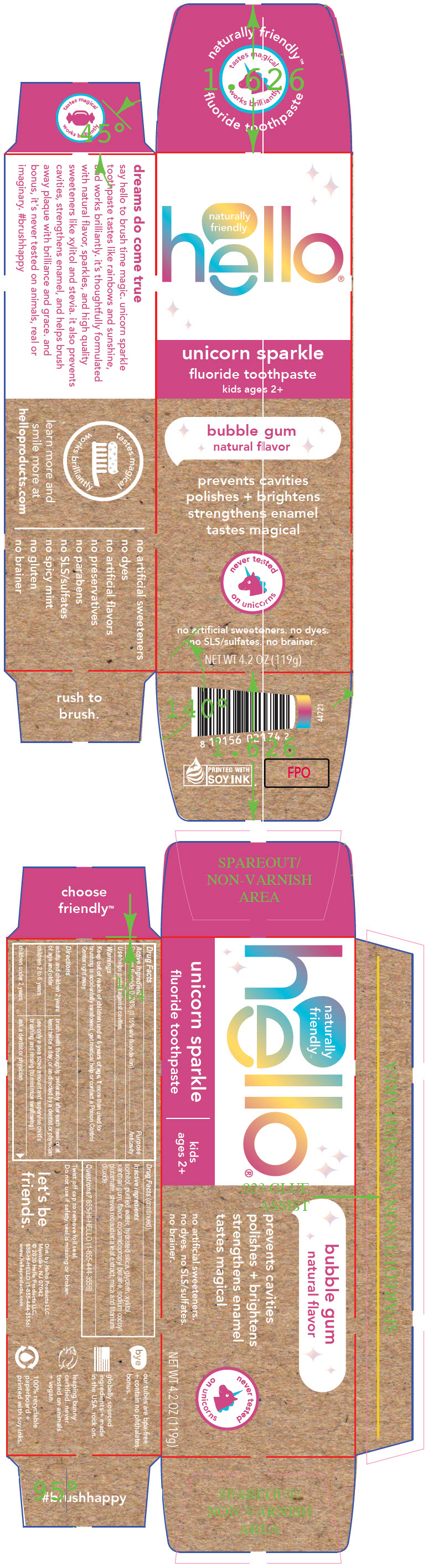
PRINCIPAL DISPLAY PANEL - 119 g Tube Carton
naturally
friendly
hello®
unicorn sparkle
fluoride toothpaste
kids ages 2+
bubble gum
natural flavor
prevents cavities
polishes + brightens
strengthens enamel
tastes magical
never tested
on unicorns
no artificial sweeteners. no dyes.
no SLS/sulfates. no brainer.
NET WT 4.2 OZ (119g)
| HELLO UNICORN
sodium fluoride gel, dentifrice |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Hello Products LLC (040714890) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.