ACID REDUCER- ranitidine tablet
acid reducer by
Drug Labeling and Warnings
acid reducer by is a Otc medication manufactured, distributed, or labeled by McKesson, Ranbaxy Pharmaceuticals Inc., Shasun Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
-
WARNINGS
Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
Do Not Use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
-
DIRECTIONS
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- children under 12 years: ask a doctor
- adults and children 12 years and over:
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
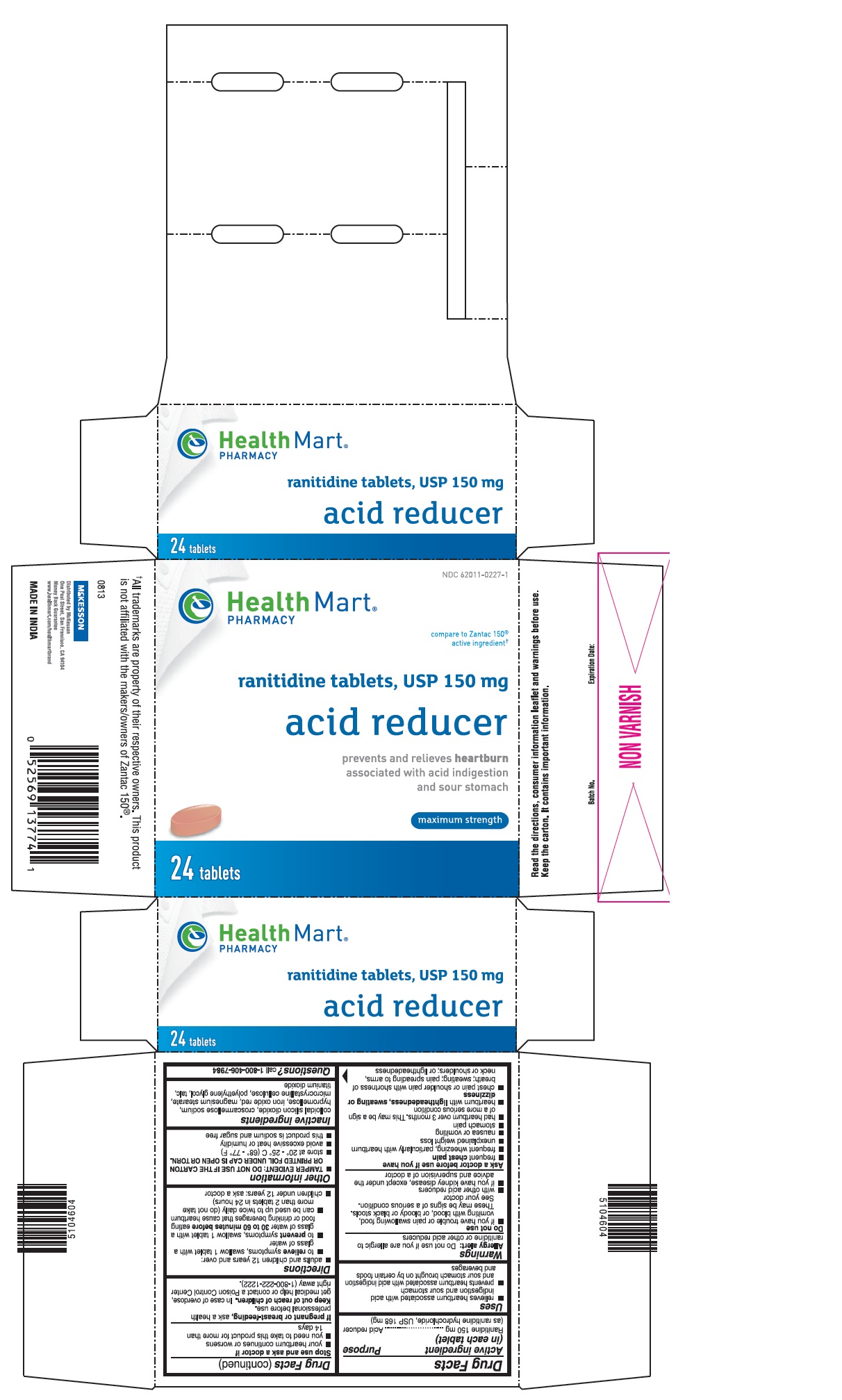
PRINCIPAL DISPLAY PANEL
NDC: 62011-0227-1
compare to Zantac 150® active ingredient†
ranitidine tablets, USP 150 mg
prevents and relieves heartburn associated with acid indigestion and sour stomach
-
INGREDIENTS AND APPEARANCE
ACID REDUCER
ranitidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62011-0227 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL (Oval Shaped) Size 12mm Flavor Imprint Code 9R Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62011-0227-1 1 in 1 CARTON 1 24 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200536 01/07/2014 Labeler - McKesson (177667227) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Shasun Pharmaceuticals Limited 915786829 manufacture(62011-0227)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.