PUR-WASH- water solution
PUR-WASH by
Drug Labeling and Warnings
PUR-WASH by is a Otc medication manufactured, distributed, or labeled by Niagara Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
-
Directions
pull cover off cap
avoid contamination of rim and inside surfaces of the eyecup
place eyecup surface to the affected eye, pressing tightly to prevent the escape of the liquid and tilt the head backward
open eyelids wide and rotate eyeball while controlling the rate of flow of solution by pressure on the bottle to ensure thorough bathing with the wash
- Other information
- Inactive ingredients
- Questions ?
- SPL UNCLASSIFIED SECTION
-
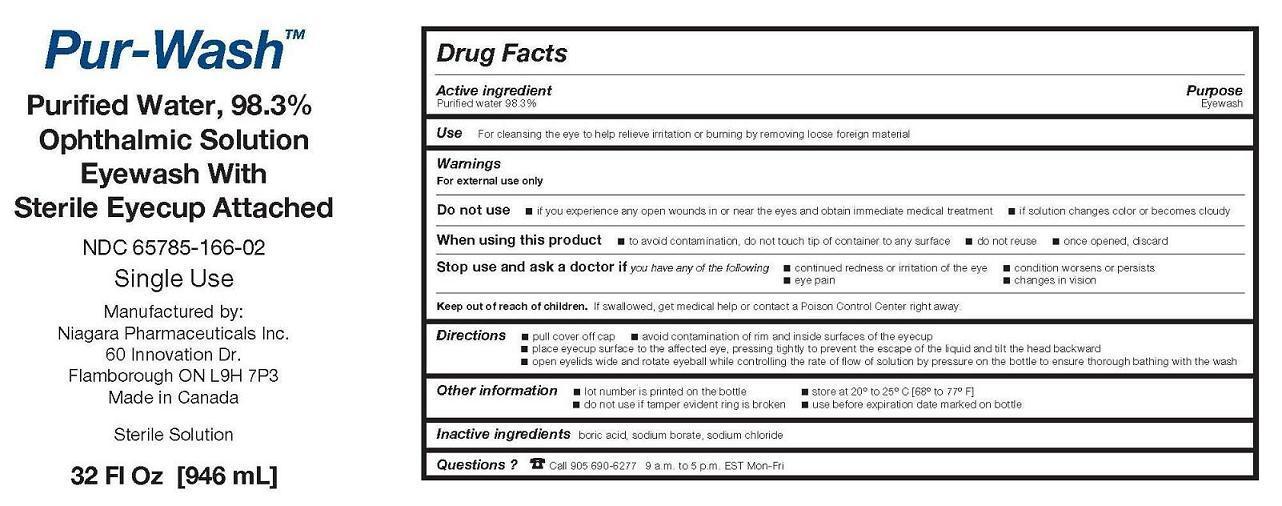
PRINCIPAL DISPLAY PANEL - 946 mL Bottle Label
Pur-Wash TM
Purified Water, 98.3%
Ophthalmic Solution
Eyewash With
Sterile Eyecup AttachedNDC: 65785-166-02
Single Use
Manufactured by:
Niagara Pharmaceuticals Inc.
60 Innovation Dr.
Flamborough ON L9H 7P3
Made in CanadaSterile Solution
32 Fl Oz [946 mL]
-
INGREDIENTS AND APPEARANCE
PUR-WASH
water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65785-166 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 929 g in 946 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM BORATE (UNII: 91MBZ8H3QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65785-166-01 473 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 05/24/2013 2 NDC: 65785-166-02 946 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 05/24/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 05/24/2013 Labeler - Niagara Pharmaceuticals Inc. (205477792) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals Inc. 205477792 manufacture(65785-166)
Trademark Results [PUR-WASH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PUR-WASH 88122071 not registered Live/Pending |
Niagara Pharmaceuticals Inc. 2018-09-18 |
 PUR-WASH 85433300 4401858 Live/Registered |
Niagara Pharmaceuticals Inc. 2011-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.