FLOR-OPAL SUSTAINED-RELEASE FLUORIDE- sodium fluoride gel
Flor-Opal Sustained-Release Fluoride by
Drug Labeling and Warnings
Flor-Opal Sustained-Release Fluoride by is a Prescription medication manufactured, distributed, or labeled by Ultradent Products, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Description
- Indications
-
Procedure
- 1. Read and understand instructions before using or dispensing to patients.
- 2.
Fabricate tray to meet patient's needs. See laboratory instructions (page 2) or send the working cast/model and prescription order form to Ultradent. Areas of sensitivity should be indicated on the cast before sending the model. If patient already has a custom bleaching tray, instruct them to apply Flor-Opal in the same manner as the bleaching gel.
Note: Trays made only for Flor-Opal may be constructed without scalloping; however, one should scallop trays made for Opalescence® bleaching.

- 3. Flor-Opal is dispensed in 1.2ml unit dose syringes. An application of a complete arch should use most of the contents of one unit dose syringe. Tray is loaded by running gel on underside of occlusal and incisal areas.
- 4. The length and number of times the tray is worn depends on the condition, the patient, and the clinician. Recommended treatment times generally range from 2 to 8 hours. Wearing the tray at night may be suggested since the gel generally stays active in the tray 8-10 hours because of slower muscle and salivary activity at night. Gel usually lasts 4-6 hours with daytime activity.
- 5.
Tray should be removed at meal times. Following tray removal, patient should brush and rinse with water. DO NOT swallow solution during rinsing. Wash tray and store in a tray storage case. Fresh gel should be used for each application.
- 6. Gel may be warmed before placing in tray to avoid thermal sensitivity.
Fig. 1
Fig. 2
-
Laboratory Instructions
- 1.
Pour and trim cast as you would for a standard bleaching tray. Apply 0.5-1.0mm of Ultradent® LC Block-Out Resin to a clean, dry cast.
- 2. Cure LC Block-Out for ~2 minutes in a light curing unit. A hand-held intraoral light (VALO® or another high quality curing light (output > 600mw/cm2)) can be used. Light cure 20 seconds using a scanning motion over the arch. Carefully check resin cure by tapping the surface of the resin with an instrument. Wipe off oxygen inhibition layer.
- 3. With vacuum former (Ultra-Form® or EconoForm™), heat a Sof-Tray® sheet until it sags ~1 inch. Activate vacuum and adapt softened plastic over mold. Cool and remove model.
- 4. Cut excess bulk of material away with Ultra-Trim™ scalloping scissors.
- 5. Trim excess tray material. Contour the tray margin to avoid frenum attachments, tori, and similar anatomical structures.
- 6. Return tray to model; check tray extensions. Gently flame polish edges one quadrant at a time (Blazer® Micro Torch).
- 7. While still warm, hold periphery of each segment firmly against model for 3 seconds with water-moistened finger. If an area is short of the desired length, gently heat and push the tray material to the desired location. If this material thins too much, a new tray should be fabricated.
- 2. Cure LC Block-Out for ~2 minutes in a light curing unit. A hand-held intraoral light (VALO® or another high quality curing light (output > 600mw/cm2)) can be used. Light cure 20 seconds using a scanning motion over the arch. Carefully check resin cure by tapping the surface of the resin with an instrument. Wipe off oxygen inhibition layer.
- 1.
Pour and trim cast as you would for a standard bleaching tray. Apply 0.5-1.0mm of Ultradent® LC Block-Out Resin to a clean, dry cast.
-
Precautions
- 1.
If any problems occur, have the patient discontinue treatment and notify the dentist.
- 2. Patients should not swallow large volumes of Flor-Opal gel. Excess at insertion and residue upon removal should be brushed off and rinsed; DO NOT swallow.
- 3. Keep out of heat and sunlight at all times. Flor-Opal should be stored at room temperature.
- 4. Not intended for use by pregnant women.
- 2. Patients should not swallow large volumes of Flor-Opal gel. Excess at insertion and residue upon removal should be brushed off and rinsed; DO NOT swallow.
EN- DENTIST INSTRUCTIONS & LAB INSTRUCTIONS
Construct and dry the stone model, cutting palate and tongue areas out. 
Vacuum adapt Sof-Tray® .035˝ sheet to model. Cool and separate. 
For sensitive roots use Ultradent's LC Block-Out Resin on gingival margin to provide a reservoir space for Flor-Opal. It is recommended to lap tray 2-3mm onto gingiva. General Precautions
- 1. For professional use only.
- 2. Review instructions, precautions, and MSDS before beginning treatment. Use only as directed.
- 3. Keep products out of heat/sunlight.
- 4. Avoid skin exposure to resins.
- 5. Isolate strong chemicals to area of treatment.
- 6. Confirm that patient has no known allergies to treatment materials.
- 7. Test flow of materials from syringe and tip before using intraorally.
- 8. Never force syringe plungers.
- 9. Clean and disinfect syringes between patients.
EN- PATIENT INSTRUCTIONS
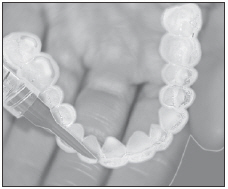
Load gel into your bleaching tray made by your dentist. Use 1/3 to 1/2 of syringe. 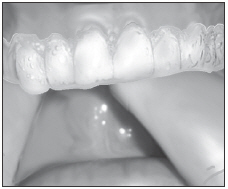
Brush teeth, then insert tray. Adapt tray sides to teeth. 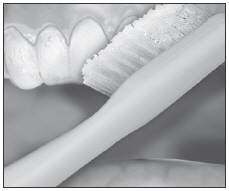
Remove excess gel with clean finger or soft toothbrush. Rinse twice; do not swallow rinses. 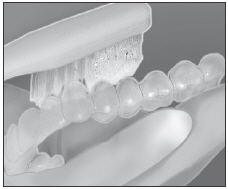
Clean tray with soft brush and cool tap water. Store tray in case provided. - 1.
If any problems occur, have the patient discontinue treatment and notify the dentist.
-
SPL UNCLASSIFIED SECTION
Use by date 
See instructions 
Recycle 
Lot Number 
Keep out of reach of children 
Store at room temperature 
Health Hazard 
EN For Professional use only. Not for injection.
Ultradent syringes have an expiration date stamped on the side of the syringe consisting of one letter and three numbers.
The letter is a lot number used for manufacturing purposes and the three numbers are the expiration date.
The first two numbers are the month, and the third number is the last number of the year.Flor-Opal
For product SDS please see our
website: www.ultradent.com
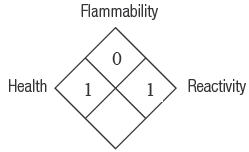
1-800-552-5512; 801-572-4200Hazard Rating
4 = Severe
3 = Serious
2 = Moderate
1 = Slight
0 = MinimalFor professional use only.
U.S. federal law restricts the sale of this device by or on the order of a dentist.
Keep out of reach of children.For immediate reorder and/or complete descriptions of Ultradent's product line, refer to Ultradent's catalog or call Toll Free 1-800-552-5512.
Outside U.S. call (801) 572-4200
or visit www.ultradent.com.© Copyright 2017 Ultradent Products, Inc. All Rights Reserved.
ULTRADENT
PRODUCTS, INC.Manufatured by Ultradent Products, Inc.
505 West Ultradent Drive (10200 South)
South Jordan, Utah 84095, USA Made in USAUltradent Products GmbH
Am Westhover Berg 30
51149 Cologne Germany10115.10 040517
- PRINCIPAL DISPLAY PANEL - 1.2 ml Syringe Package
-
INGREDIENTS AND APPEARANCE
FLOR-OPAL SUSTAINED-RELEASE FLUORIDE
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51206-401 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Carbomer Homopolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: HHT01ZNK31) Glycerin (UNII: PDC6A3C0OX) Polyethylene Glycol 300 (UNII: 5655G9Y8AQ) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51206-401-01 20 in 1 PACKAGE 05/31/1990 01/31/2023 1 1.2 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 05/31/1990 01/31/2023 Labeler - Ultradent Products, Inc (013369913) Establishment Name Address ID/FEI Business Operations Ultradent Products, Inc 013369913 MANUFACTURE(51206-401) , LABEL(51206-401) , ANALYSIS(51206-401) , PACK(51206-401)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
