STERILE TALC- talc powder
Sterile Talc by
Drug Labeling and Warnings
Sterile Talc by is a Prescription medication manufactured, distributed, or labeled by Bryan Corporation, Sciarra Laboratories, Sciarra Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use STERILE TALC POWDER safely and effectively. See full prescribing information for STERILE TALC POWDER.
STERILE TALC POWDER (talc), for intrapleural use Initial U.S. Approval: 2003INDICATIONS AND USAGE
STERILE TALC POWDER is a sclerosing agent indicated to decrease the recurrence of malignant pleural effusions in symptomatic patients following maximal drainage of the pleural effusion. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
5 g powder in a single-dose bottle, for suspension (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Pneumonitis and Acute Respiratory Distress Syndrome (ARDS): Acute Pneumonitis and ARDS, including fatal cases, occur with intrapleural talc administration. (5.1)
- Interference with Future Procedures: Sclerosis of the pleural space may preclude or complicate subsequent ipsilateral surgery and diagnostic procedures. (5.2)
ADVERSE REACTIONS
Commonly observed adverse reactions are fever and pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bryan Corporation at 1-800-343-7711 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pneumonitis and Acute Respiratory Distress Syndrome (ARDS)
5.2 Interference with Future Procedures
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose is 5 g, suspended in 50 ml to 100 ml 0.9% Sodium Chloride Injection, USP
2.2 Preparation
Prepare the talc suspension using aseptic technique in an appropriate laminar flow hood as follows:
Step 1. Using a 16 gauge needle attached to a 60-ml LuerLok syringe, draw up 50 ml of 0.9 % Sodium Chloride injection, USP. Vent the talc bottle using a needle. Slowly inject the 50 ml of 0.9% Sodium Chloride Injection, USP into the bottle.
Step 2. Swirl the bottle to disperse the talc powder.
Step 3. Divide the contents of the bottle equally into two 60 ml LuerLok syringes, each attached with a 16 gauge needle, by withdrawing 25 ml of the suspension into each syringe with continuous swirling. Add 0.9% Sodium Chloride Injection, USP to a total volume of 50 ml in each syringe. Draw 10 ml of air into each syringe to the 60 ml mark to serve as a headspace for mixing prior to administration. Each syringe should contain 2.5 g of Sterile Talc Powder in 50 ml of 0.9% Sodium Chloride Injection, USP with an air headspace of 10 ml.
Step 4. Label the syringes with the talc concentration, the expiration date and time, the identity of the patient intended to receive the material, and the following statements:
“SHAKE SYRINGE WELL to resuspend before administration”
“FOR PLEURODESIS ONLY – not for intravenous administration”
Step 5. If not used immediately, store prepared suspension in refrigerator. Discard the prepared suspension if not used within 12 hours.
2.3 Administration
Prior to administration, continuously agitate the syringes to evenly redisperse the talc and avoid settlement. Immediately prior to administration, vent the 10 ml air headspace from each syringe. Administer the talc suspension through the chest tube according to standard procedures.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ADVERSE REACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B.
Risk Summary
A reproduction study performed in rabbits at doses up to approximately 5 times the human dose revealed no evidence of teratogenicity. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Animal Data
Rabbits were administered talc by oral gavage daily during the period of organogenesis at doses of up to 900 mg/kg (approximately 5 times the human dose on a mg/m2 basis). No significant dose-related toxicity was reported except at maternally toxic doses. In multiple animal studies, intrapleurally administered talc was not absorbed systemically.
-
11 DESCRIPTION
STERILE TALC POWDER is a sclerosing agent for intrapleural administration. STERILE TALC POWDER is white or off-white to light gray, asbestos-free and brucite-free sterile talc powder of controlled particle size. The powder is ≥ 95% hydrated magnesium silicate [Mg3Si4O10 (OH)2, molecular weight 379.3]; associated minerals include chlorite (hydrated aluminum and magnesium silicate), dolomite (calcium and magnesium carbonate), calcite (calcium carbonate), and quartz. Talc is insoluble in water.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies on the carcinogenicity of talc have been performed using non-standard designs which prevent firm conclusions on its carcinogenicity. With single intraperitoneal administration to mice at 20 mg and observation for at least 6 months or 4 weekly doses administered intraperitoneally at 25 mg/dose to rats with observation for at least 84 weeks, tumor incidence was not increased. In these studies the talc and its asbestos content were not characterized.
Genotoxicity was tested in cultures of rat pleural mesothelial cells (RPMC) as unscheduled DNA synthesis (UDS) and sister chromatid exchanges (SCEs). None of the talc samples (which were asbestos-free) induced enhancement of UDS or SCEs in treated cultures. No information is available on impairment of fertility in animals by talc.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
STERILE TALC POWDER is supplied in a single use 100 ml brown glass bottle, sealed with a gray, 20 mm stopper and covered with a flip-off seal.
NDC: 63256-200-05: 5 gram individual bottle packaged in a pouch.
NDC: 63256-200-10: Carton of ten (10) 5-gram bottles.
Store the powder at 25°C (77°F); excursions permitted between15°C to 30°C (59°F - 86°F) [see USP Controlled Room Temperature]. Protect against sunlight.
-
17 PATIENT COUNSELING INFORMATION
Advise patients to notify their healthcare provider if new or worsening pulmonary symptoms develop [see Warnings and Precautions (5.1)].
Distributed by:
Bryan Corporation. Woburn, MA 01801. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Bottle Label
Sterile Talc Powder™
(Talc) Powder
Contains 5 grams of sterile talc per bottle
For Intrapleural Administration
Refer to the Prescribing Information for dosage and preparation.Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F - 86°F)
[see USP Controlled Room Temperature]. Protect against sunlight.Distributed By: Bryan Corporation Lot#
Woburn, MA 01801 Expired:Rx Only

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Pouch Label
Sterile Talc Powder™ NDC: 63256-200-05
(Talc) Powder
Contains 5 grams of sterile talc per bottle 1 Bottle
For Intrapleural Administration
Refer to the Prescribing Information for dosage and preparation.Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F - 86°F)
[see USP Controlled Room Temperature]. Protects against sunlight.Distributed By: Bryan Corporation Lot#
Woburn, MA 01801 Expired:Rx Only

-
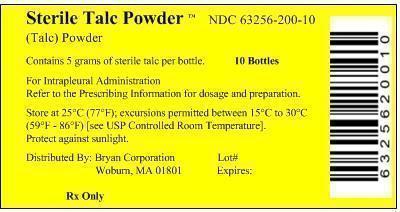
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Box Label
Sterile Talc Powder™ NDC: 63256-200-10
(Talc) Powder
Contains 5 grams of sterile talc per bottle 10 Bottles
For Intrapleural Administration
Refer to the Prescribing Information for dosage and preparation.Store at 25°C (77°F); excursions permitted between 15°C to 30°C
(59°F - 86°F) [see USP Controlled Room Temperature].Distributed By: Bryan Corporation Lot#
Woburn, MA 01801 Expired:Rx Only
-
INGREDIENTS AND APPEARANCE
STERILE TALC
talc powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63256-200 Route of Administration INTRAPLEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Talc (UNII: 7SEV7J4R1U) (Talc - UNII:7SEV7J4R1U) Talc 5 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63256-200-10 10 in 1 BOX 1 100 mL in 1 BOTTLE 2 NDC: 63256-200-05 1 in 1 POUCH 2 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021388 12/15/2003 Labeler - Bryan Corporation (147033401) Registrant - Sciarra Laboratories (824900369) Establishment Name Address ID/FEI Business Operations Sciarra Laboratories, Inc. 824900369 MANUFACTURE(63256-200)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.