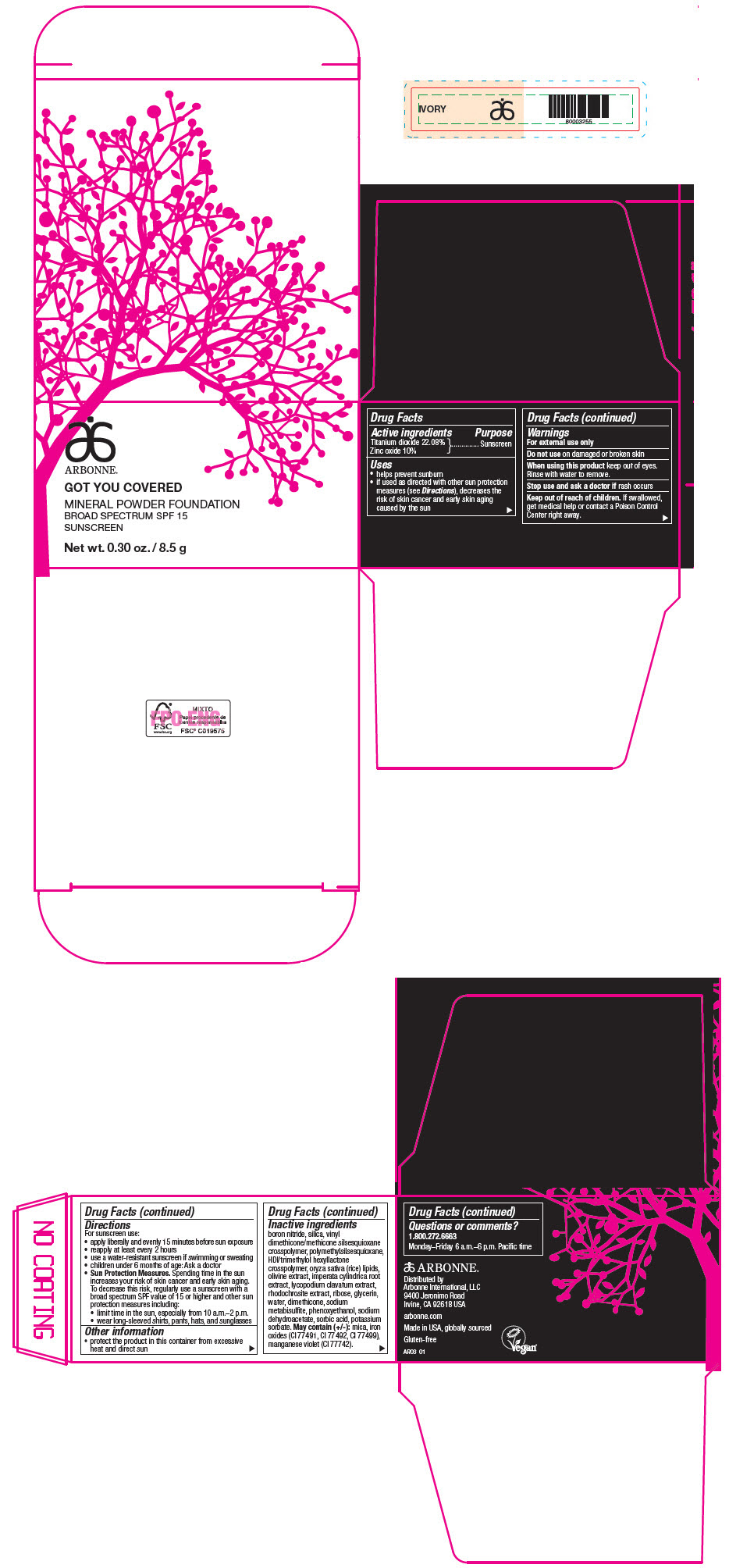
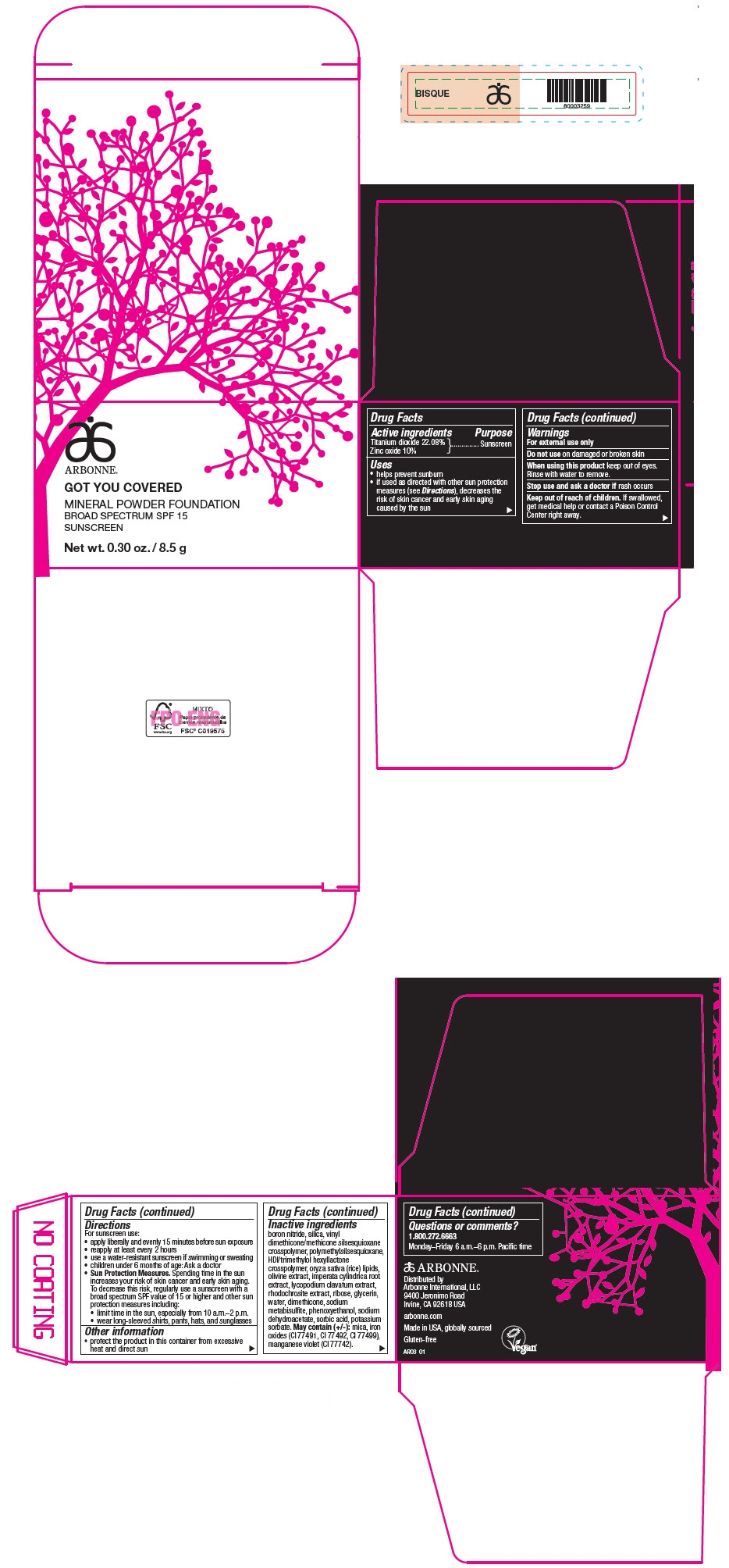
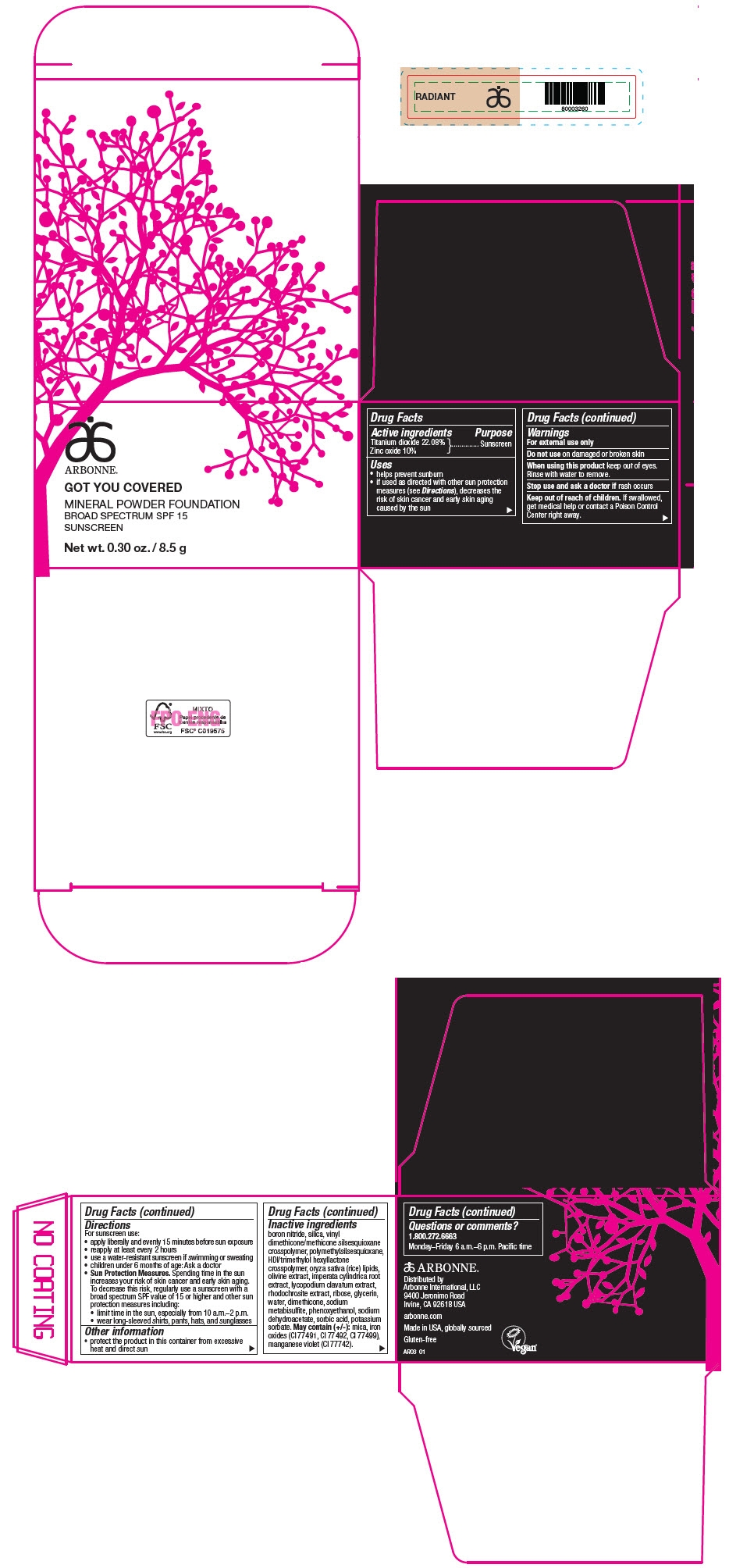
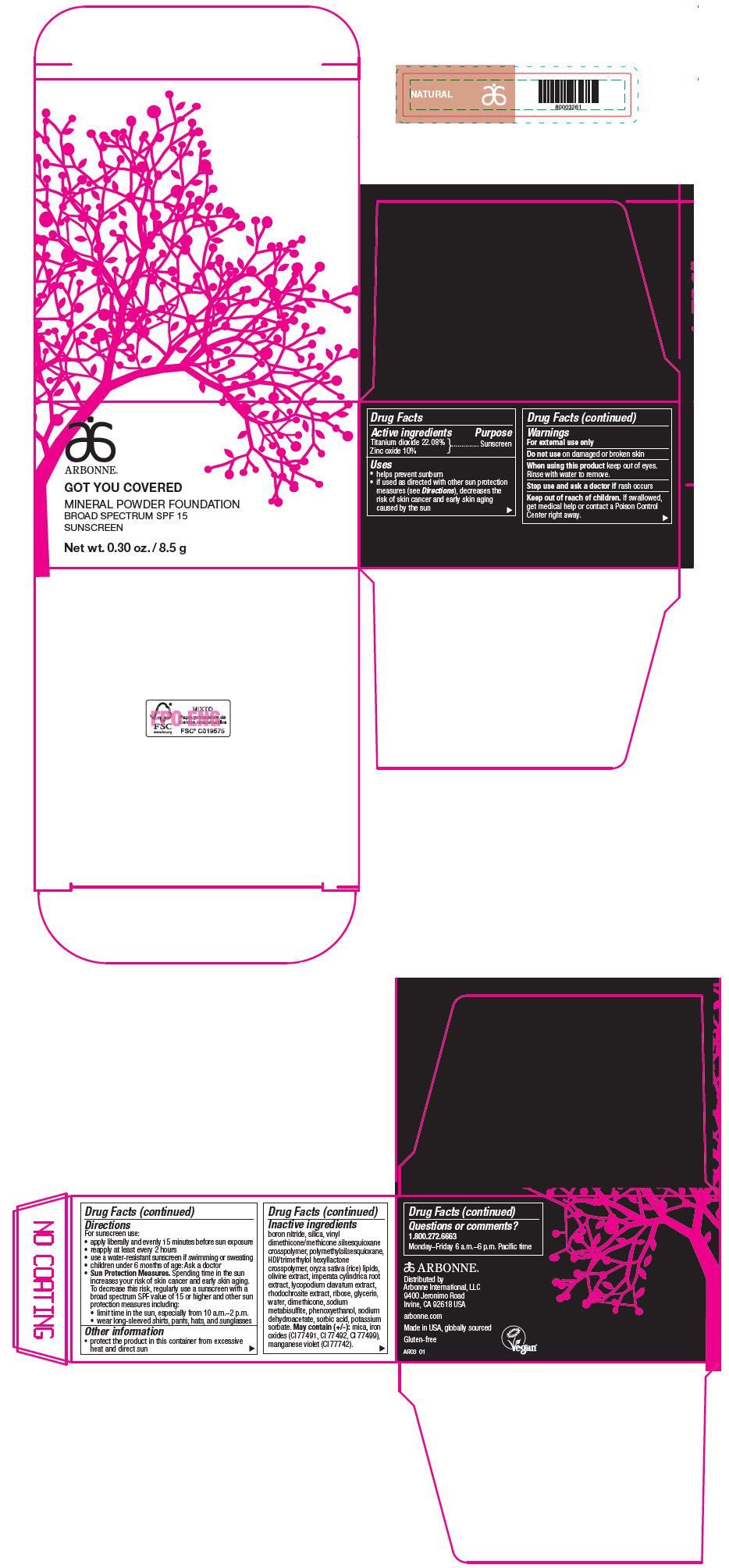
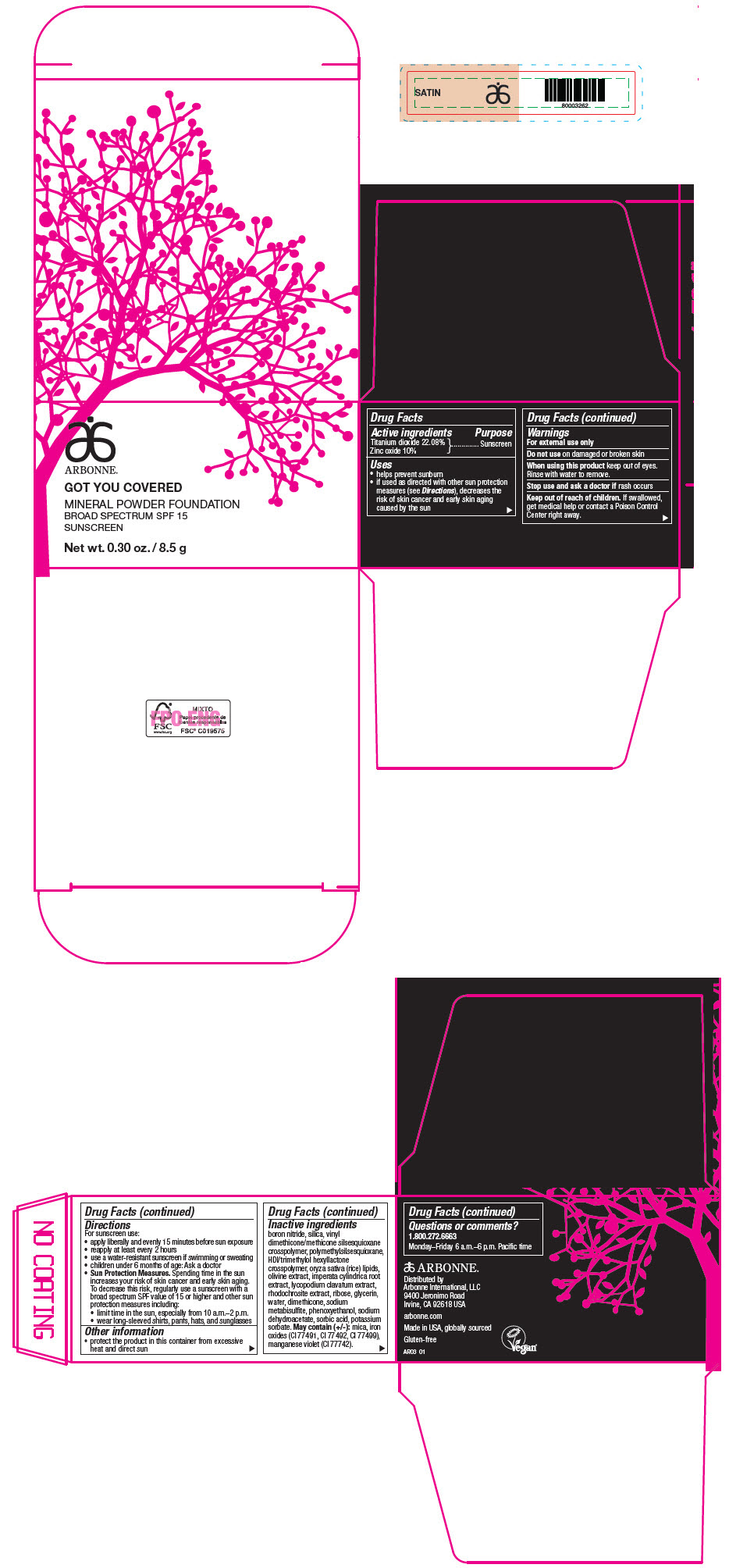
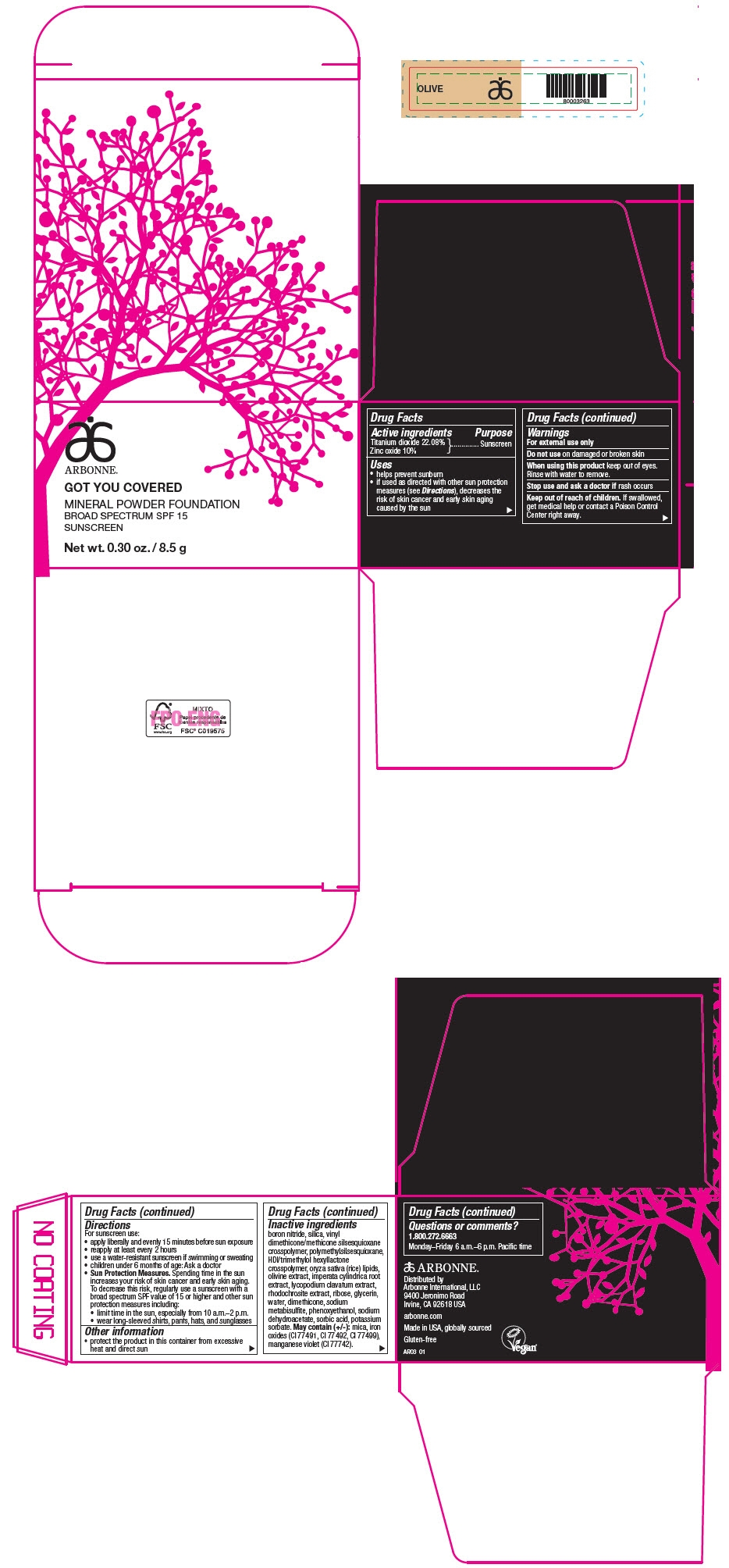
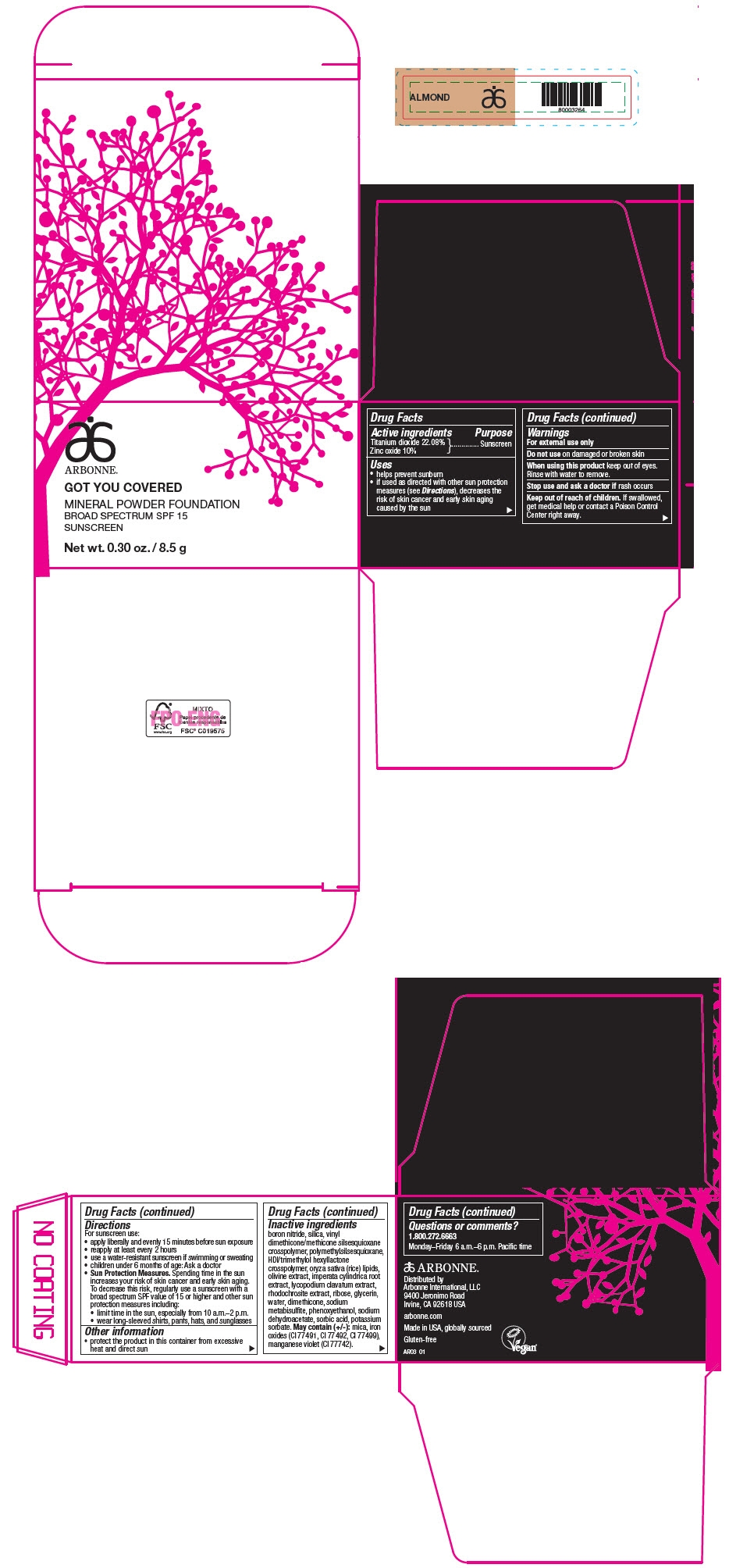
Active ingredients
Titanium dioxide 22.08%
Zinc oxide 10%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Other information
- protect the product in this container from excessive heat and direct sun
Inactive ingredients
boron nitride, silica, vinyl dimethicone/methicone silsesquioxane crosspolymer, polymethylsilsesquioxane, HDI/trimethylol hexyllactone crosspolymer, oryza sativa (rice) lipids, olivine extract, imperata cylindrica root extract, lycopodium clavatum extract, rhodochrosite extract, ribose, glycerin, water, dimethicone, sodium metabisulfite, phenoxyethanol, sodium dehydroacetate, sorbic acid, potassium sorbate. May contain (+/-): mica, iron oxides (CI 77491, CI 77492, CI 77499), manganese violet (CI 77742).
Questions or comments?
1.800.272.6663
Monday–Friday 6 a.m.–6 p.m. Pacific time
Distributed by
Arbonne International, LLC
9400 Jeronimo Road
Irvine, CA 92618 USA
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - IVORY
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
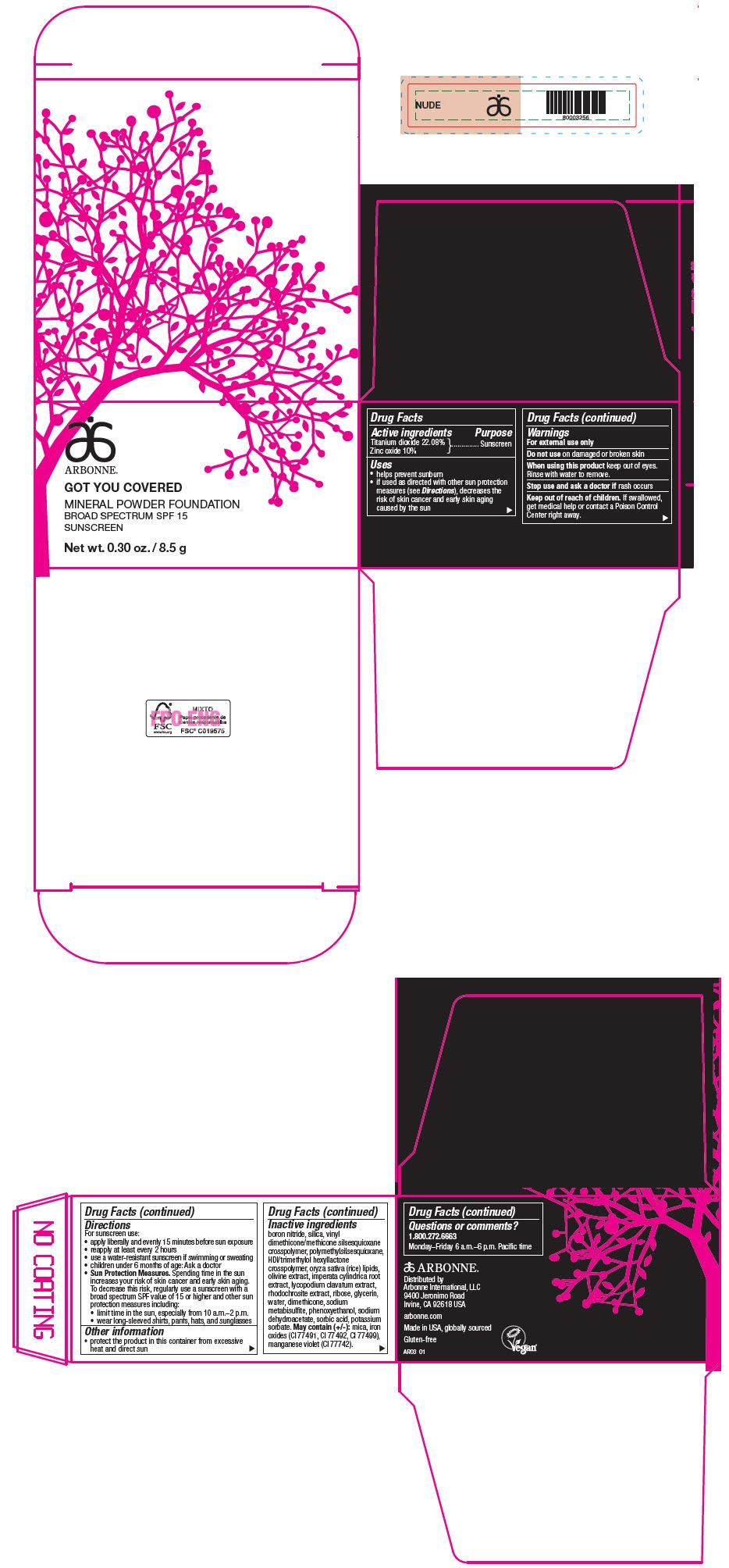
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - NUDE
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
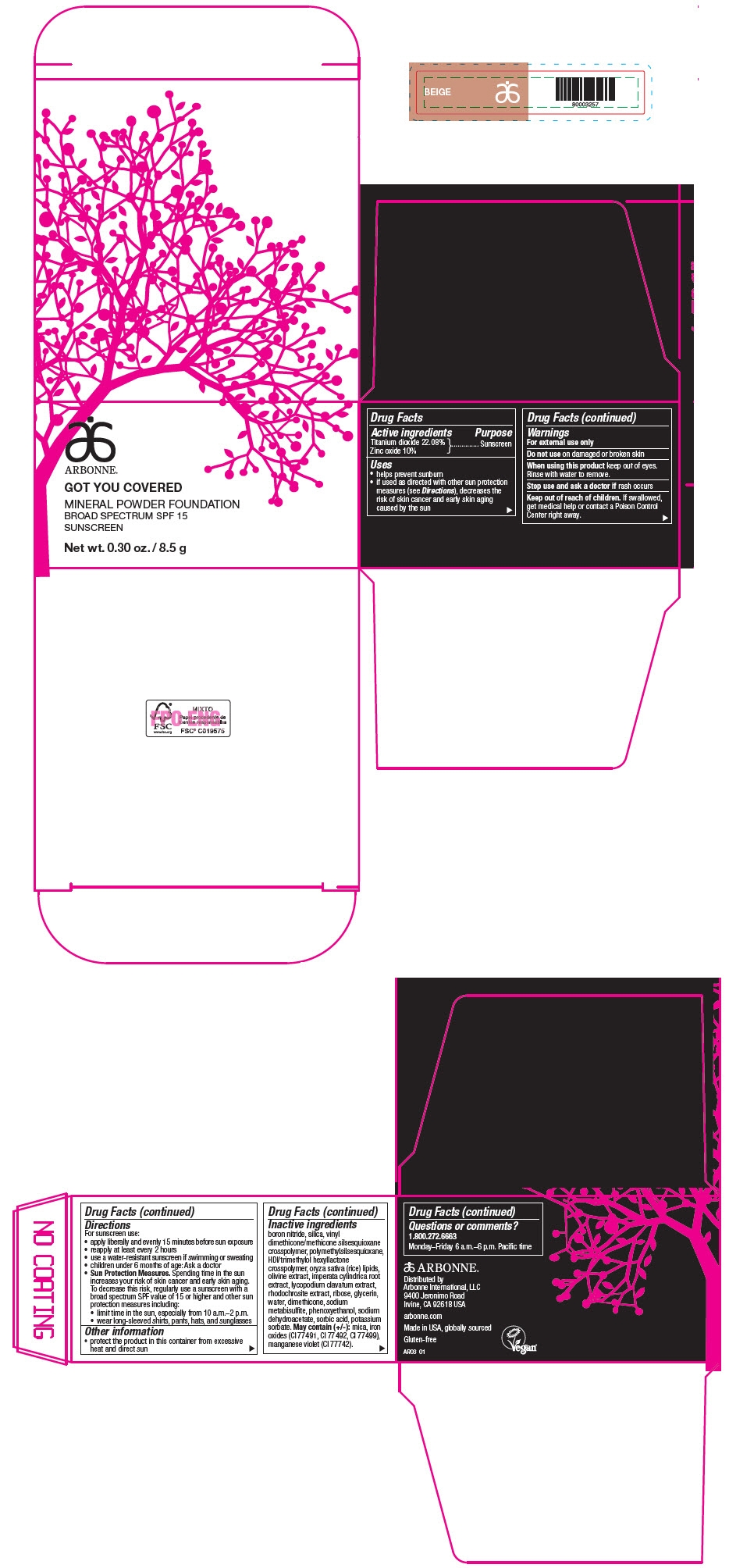
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - BEIGE
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
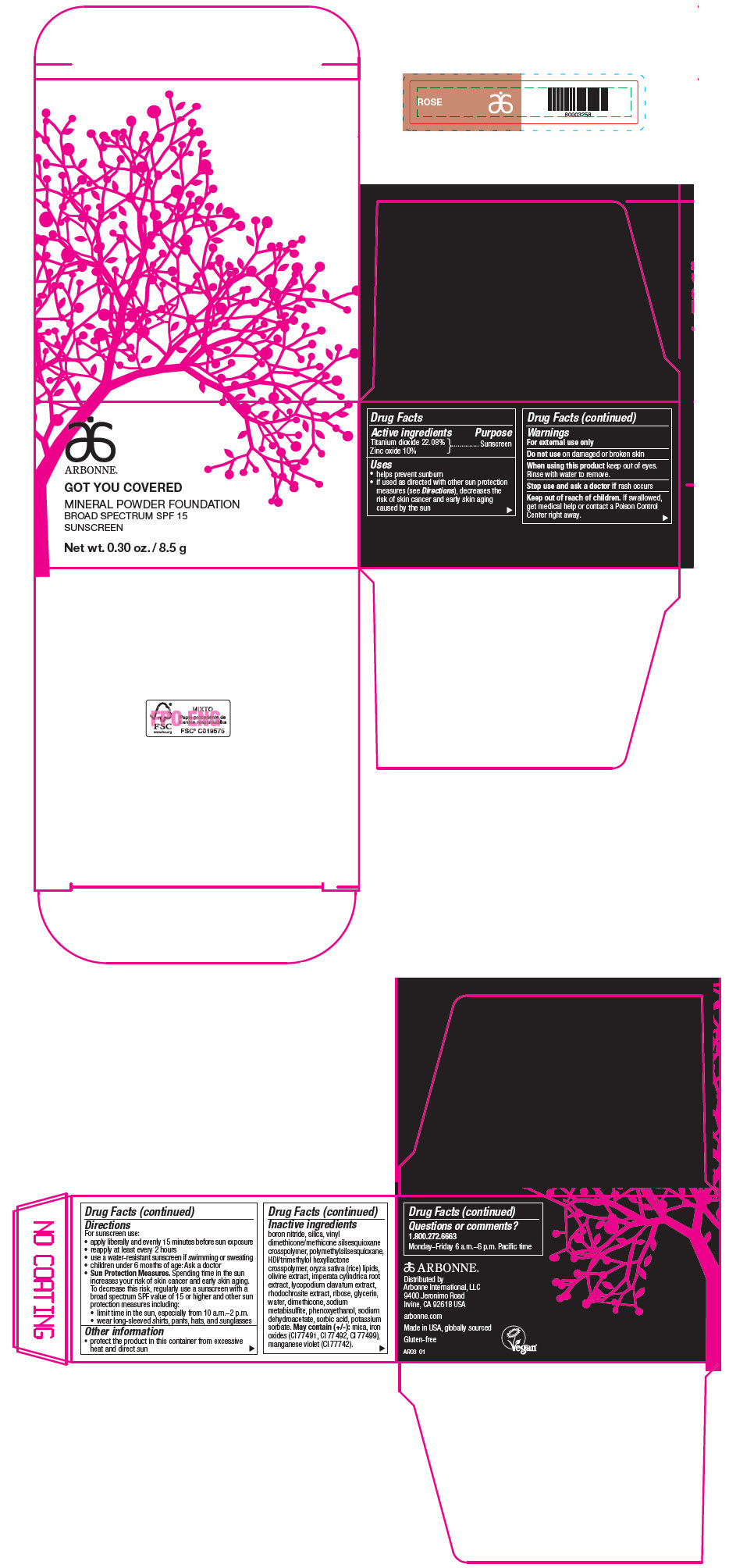
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - ROSE
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - BISQUE
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - RADIANT
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - NATURAL
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - SATIN
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - OLIVE
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g
PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton - ALMOND
ARBONNE®
GOT YOU COVERED
MINERAL POWDER FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
Net wt. 0.30 oz. / 8.5 g