DAYQUIL AND NYQUIL SEVERE PLUS VICKS VAPOCOOL COLD AND FLU- acetaminophen, phenylephrine hydrochloride, and doxylamine succinate kit
DayQuil and Nyquil Severe plus Vicks VapoCool by
Drug Labeling and Warnings
DayQuil and Nyquil Severe plus Vicks VapoCool by is a Otc medication manufactured, distributed, or labeled by The Procter & Gamble Manufacturing Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients 9in each caplet)
-
Uses
- temporarily relieves common cold/flu symptoms:
- nasal congestion
- sinus congestion & pressure
- cough due to minor throat & bronchial irritation
- minor aches & pains
- headache
- fever
- sore throat
- reduces swelling of nasal passages
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
-
Warnings
Liver warning
This product contains acetaminophen.
Severe liver damage may occur if you take
- more than 8 caplets in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy Alert:
Acetaminophen may cause severe skin reactions. Symptoms may include:
- Skin reddening
- Blisters
- Rash
If a skin reaction occurs, stop use and seek medical help right away
Sore throat warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each caplet)
-
Uses
temporarily relieves common cold/flu symptoms:
- nasal congestion
- sinus congestion & pressure
- cough due to minor throat & bronchial irritation
- cough to help you sleep
- minor aches & pains
- headache
- fever
- sore throat
- runny nose & sneezing reduces swelling of nasal passages
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
-
Warnings
Liver warning
This product contains acetaminophen.
Severe liver damage may occur if you take
- more than 8 caplets in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy Alert:
acetaminophen may cause severe skin reactions.
Symptoms may include:- Skin reddening
- Blisters
- Rash
If a skin reaction occurs, stop use and seek medical help right away
Sore throat warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- to make a child sleep
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema
- trouble urinating due to enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives & tranquilizers may increase drowsiness
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
- Directions
- Other information
-
Inactive ingredients
crospovidone, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, flavor, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, pregelatinized starch, silicon dioxide, stearic acid, sucralose, talc, titanium dioxide
- Questions?
- SPL UNCLASSIFIED SECTION
-
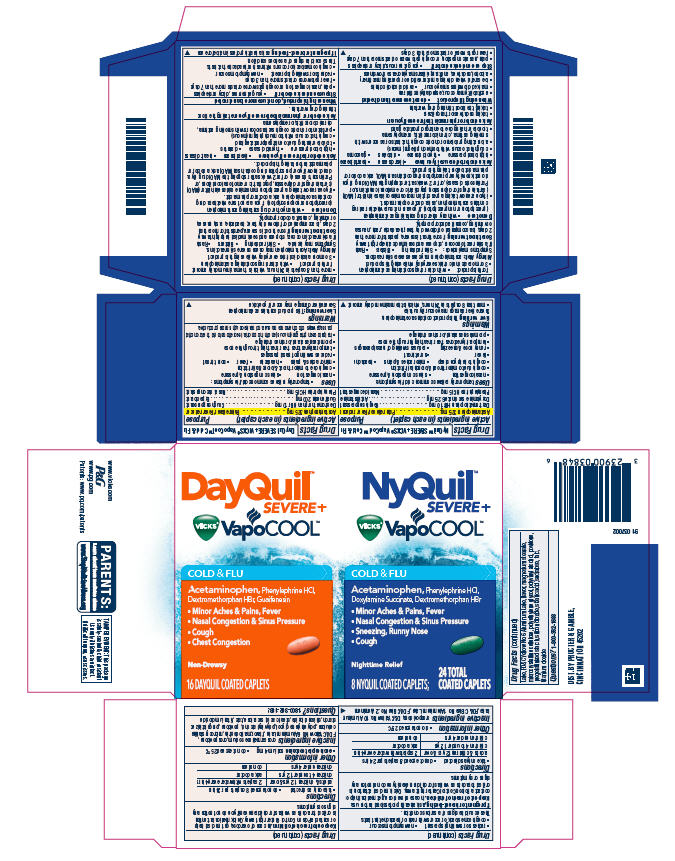
PRINCIPAL DISPLAY PANEL - Kit Carton
DayQuil™
SEVERE +
VICKS® VapoCOOL™
COLD & FLU
Acetaminophen, Phenylephrine HCl,
DextromethorphanHBr,Guaifenesin
- Minor Aches & Pains, Fever
- Nasal Congestion & Sinus Pressure
- Cough
- Chest Congestion
Non-Drowsy
32 DAYQUIL COATED CAPLETS
NyQuil™
SEVERE +
VICKS® VapoCOOL™
COLD & FLU
Acetaminophen, Phenylephrine HCl,
DoxylamineSuccinate, DextromethorphanHBr
- Minor Aches & Pains, Fever
- Nasal Congestion & Sinus Pressure
- Sneezing, Runny Nose
- Cough
Nighttime Relief16 NYQUIL COATED CAPLETS; 48 TOTAL COATED CAPLETS
-
INGREDIENTS AND APPEARANCE
DAYQUIL AND NYQUIL SEVERE PLUS VICKS VAPOCOOL COLD AND FLU
acetaminophen, phenylephrine hydrochloride, and doxylamine succinate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37000-534 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37000-534-24 1 in 1 PACKAGE; Type 1: Convenience Kit of Co-Package 08/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 16 BLISTER PACK 16 Part 2 8 BLISTER PACK 8 Part 1 of 2 DAYQUIL SEVERE PLUS VICKS VAPOCOOL COLD AND FLU
acetaminophen, dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride tabletProduct Information Item Code (Source) NDC: 37000-524 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MALTODEXTRIN (UNII: 7CVR7L4A2D) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STEARIC ACID (UNII: 4ELV7Z65AP) CROSPOVIDONE (UNII: 2S7830E561) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TALC (UNII: 7SEV7J4R1U) SUCRALOSE (UNII: 96K6UQ3ZD4) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color orange Score no score Shape BULLET Size 19mm Flavor Imprint Code DQ Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2018 Part 2 of 2 NYQUIL SEVERE PLUS VICKS VAPOCOOL COLD AND FLU
acetaminophen, phenylephrine hydrochloride, doxylamine succinate, and dextromethorphan hydrobromide tablet, coatedProduct Information Item Code (Source) NDC: 37000-523 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) STEARIC ACID (UNII: 4ELV7Z65AP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) SUCRALOSE (UNII: 96K6UQ3ZD4) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color green Score no score Shape BULLET Size 19mm Flavor Imprint Code NQ Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2018 Labeler - The Procter & Gamble Manufacturing Company (004238200)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.