LUTATHERA- lutetium lu 177 dotatate injection
Lutathera by
Drug Labeling and Warnings
Lutathera by is a Prescription medication manufactured, distributed, or labeled by Advanced Accelerator Applications USA, Inc, Advanced Accelerator Applications USA, Inc., Advanced Accelerator Applications (Italy) srl, Advanced Accelerator Applications (Italy) S.r.l., C.A.T. GmbH & Co. Chromatographie und Analysentechnik KG, Osterreichische Agentur fur Gesundheit and Ernahrungssicherheit Gmbh, IDB Radiopharmacy B.V., piCHEM Forschungs-und Entwicklungs GmbH, University of Missouri System. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LUTATHERA safely and effectively. See full prescribing information for LUTATHERA.
LUTATHERA® (lutetium Lu 177 dotatate) injection, for intravenous use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
LUTATHERA is a radiolabeled somatostatin analog indicated for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults. (1)
DOSAGE AND ADMINISTRATION
- Verify pregnancy status in females of reproductive potential prior to initiating LUTATHERA. ( 2.1)
- Administer 7.4 GBq (200 mCi) every 8 weeks for a total of 4 doses. ( 2.2)
- Administer long-acting octreotide 30 mg intramuscularly 4 to 24 hours after each LUTATHERA dose and short-acting octreotide for symptomatic management. ( 2.3)
- Continue long-acting octreotide 30 mg intramuscularly every 4 weeks after completing LUTATHERA until disease progression or for up to 18 months following treatment initiation. ( 2.3)
- Premedicate with antiemetics 30 minutes before recommended amino acid solution. ( 2.3)
- Initiate recommended intravenous amino acid solution 30 minutes before LUTATHERA infusion; continue during and for 3 hours after LUTATHERA infusion. Do not reduce dose of amino acid solution if LUTATHERA dose is reduced. ( 2.3)
- Modify LUTATHERA dose based on adverse reactions. ( 2.4)
- Prepare and administer as recommended. ( 2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 370 MBq/mL (10 mCi/mL) in single-dose vial. ( 3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Risk from Radiation Exposure: Minimize radiation exposure during and after treatment with LUTATHERA consistent with institutional good radiation safety practices and patient management procedures ( 2.1, 5.1)
- Myelosuppression: Monitor blood cell counts. Withhold, reduce dose, or permanently discontinue based on severity. ( 2.4, 5.2)
- Secondary Myelodysplastic Syndrome (MDS) and Leukemia: Median time to development: MDS is 28 months; acute leukemia is 55 months. ( 5.3)
- Renal Toxicity: Advise patients to urinate frequently during and after administration of LUTATHERA. Monitor serum creatinine and calculated creatinine clearance. Withhold, reduce dose, or permanently discontinue based on severity. ( 2.3, 2.4, 5.4)
- Hepatotoxicity: Monitor transaminases, bilirubin and albumin. Withhold, reduce dose, or permanently discontinue based on severity. ( 2.4, 5.5)
- Neuroendocrine Hormonal Crisis: Monitor for flushing, diarrhea, hypotension, bronchoconstriction or other signs and symptoms. ( 5.6)
- Embryo-Fetal Toxicity: LUTATHERA can cause fetal harm. Advise females and males of reproductive potential of the potential risk to a fetus and to use effective contraception ( 5.7, 8.1, 8.3)
- Risk of Infertility: LUTATHERA may cause infertility. ( 8.3)
ADVERSE REACTIONS
Most common Grade 3-4 adverse reactions (≥ 4% with a higher incidence in LUTATHERA arm) are lymphopenia, increased GGT, vomiting, nausea, increased AST, increased ALT, hyperglycemia and hypokalemia. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Advanced Accelerator Applications USA, Inc. at 1-844-863-1930 or us-pharmacovigilance@adacap.com, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Safety Instructions
2.2 Recommended Dosage
2.3 Premedication and Concomitant Medications
2.4 Dose Modifications for Adverse Reactions
2.5 Preparation and Administration
2.6 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk from Radiation Exposure
5.2 Myelosuppression
5.3 Secondary Myelodysplastic Syndrome and Leukemia
5.4 Renal Toxicity
5.5 Hepatotoxicity
5.6 Neuroendocrine Hormonal Crisis
5.7 Embryo-Fetal Toxicity
5.8 Risk of Infertility
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Somatostatin Analogs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
11.1 Physical Characteristics
11.2 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Progressive, Well-differentiated Advanced or Metastatic Somatostatin Receptor-Positive Midgut Carcinoid Tumors
14.2 Somatostatin Receptor-Positive Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Safety Instructions
LUTATHERA is a radiopharmaceutical; handle with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions ( 5.1)] . Use waterproof gloves and effective radiation shielding when handling LUTATHERA. Radiopharmaceuticals, including LUTATHERA, should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radiopharmaceuticals, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radiopharmaceuticals.
Verify pregnancy status of females of reproductive potential prior to initiating LUTATHERA [see Use in Specific Populations ( 8.1, 8.3)] .
2.2 Recommended Dosage
The recommended LUTATHERA dose is 7.4 GBq (200 mCi) every 8 weeks for a total of 4 doses. Administer pre- and concomitant medications and administer LUTATHERA as recommended [see Dosage and Administration ( 2.3, 2.5) ].
2.3 Premedication and Concomitant Medications
Somatostatin Analogs
- Before initiating LUTATHERA: Discontinue long-acting somatostatin analogs (e.g., long-acting octreotide) for at least 4 weeks prior to initiating LUTATHERA. Administer short-acting octreotide as needed; discontinue at least 24 hours prior to initiating LUTATHERA [see Drug Interactions ( 7.1)] .
- During LUTATHERA treatment: Administer long-acting octreotide 30 mg intramuscularly between 4 to 24 hours after each LUTATHERA dose. Do not administer long-acting octreotide within 4 weeks of each subsequent LUTATHERA dose. Short-acting octreotide may be given for symptomatic management during LUTATHERA treatment, but must be withheld for at least 24 hours before each LUTATHERA dose.
- Following LUTATHERA treatment: Continue long-acting octreotide 30 mg intramuscularly every 4 weeks after completing LUTATHERA until disease progression or for up to 18 months following treatment initiation.
Antiemetic
Administer antiemetics 30 minutes before the recommended amino acid solution.
Amino Acid Solution
Initiate an intravenous amino acid solution containing L-lysine and L-arginine (Table 1) 30 minutes before administering LUTATHERA. Use a three-way valve to administer amino acids using the same venous access as LUTATHERA or administer amino acids through a separate venous access in the patient’s other arm. Continue the infusion during, and for at least 3 hours after LUTATHERA infusion. Do not decrease the dose of the amino acid solution if the dose of LUTATHERA is reduced [see Warnings and Precautions ( 5.4)] .Table 1. Amino Acid Solution Item
Specification
Lysine HCl content
Between 18 g and 24 g
Arginine HCl content
Between 18 g and 24 g
Volume
1.5 L to 2.2 L
Osmolarity
< 1050 mOsmol
2.4 Dose Modifications for Adverse Reactions
Recommended dose modifications of LUTATHERA for adverse reactions are provided in Table 2.
Table 2. Recommended Dose Modifications of LUTATHERA for Adverse Reactions - * National Cancer Institute, Common Toxicity Criteria for Adverse Events, version 4.03
Adverse Reaction
Severity of Adverse Reaction *
Dose Modification
Thrombocytopenia [see Warnings and Precautions (5.2)]
Grade 2, 3 or 4
Withhold dose until complete or partial resolution (Grade 0 to 1).
Resume LUTATHERA at 3.7 GBq (100 mCi) in patients with complete or partial resolution. If reduced dose does not result in Grade 2, 3 or 4 thrombocytopenia, administer LUTATHERA at 7.4 GBq (200 mCi) for next dose.
Permanently discontinue LUTATHERA for Grade 2 or higher thrombocytopenia requiring a treatment delay of 16 weeks or longer.
Recurrent Grade 2, 3 or 4
Permanently discontinue LUTATHERA.
Anemia and Neutropenia [see Warnings and Precautions (5.2)]
Grade 3 or 4
Withhold dose until complete or partial resolution (Grade 0, 1, or 2).
Resume LUTATHERA at 3.7 GBq (100 mCi) in patients with complete or partial resolution. If reduced dose does not result in Grade 3 or 4 anemia or neutropenia, administer LUTATHERA at 7.4 GBq (200 mCi) for next dose.
Permanently discontinue LUTATHERA for Grade 3 or higher anemia or neutropenia requiring a treatment delay of 16 weeks or longer.
Recurrent Grade 3 or 4
Permanently discontinue LUTATHERA.
Renal Toxicity [see Warnings and Precautions (5.4)]
Defined as:
- Creatinine clearance less than 40 mL/min; calculate using Cockcroft Gault with actual body weight, or
- 40% increase in baseline serum creatinine, or
- 40% decrease in baseline creatinine clearance; calculate using Cockcroft Gault with actual body weight.
Withhold dose until complete resolution.
Resume LUTATHERA at 3.7 GBq (100 mCi) in patients with complete resolution. If reduced dose does not result in renal toxicity, administer LUTATHERA at 7.4 GBq (200 mCi) for next dose.
Permanently discontinue LUTATHERA for renal toxicity requiring a treatment delay of 16 weeks or longer.
Recurrent renal toxicity
Permanently discontinue LUTATHERA.
Hepatotoxicity [see Warnings and Precautions (5.5)]
Defined as:
- Bilirubinemia greater than 3 times the upper limit of normal (Grade 3 or 4), or
- Hypoalbuminemia less than 30 g/L with a decreased prothrombin ratio less than 70%.
Withhold dose until complete resolution.
Resume LUTATHERA at 3.7 GBq (100 mCi) in patients with complete resolution. If reduced LUTATHERA dose does not result in hepatotoxicity, administer LUTATHERA at 7.4 GBq (200 mCi) for next dose.
Permanently discontinue LUTATHERA for hepatotoxicity requiring a treatment delay of 16 weeks or longer.
Recurrent hepatotoxicity
Permanently discontinue LUTATHERA.
Other Non-Hematologic Toxicity
Grade 3 or 4
Withhold dose until complete or partial resolution (Grade 0 to 2).
Resume LUTATHERA at 3.7 GBq (100 mCi) in patients with complete or partial resolution. If reduced dose does not result in Grade 3 or 4 toxicity, administer LUTATHERA at 7.4 GBq (200 mCi) for next dose.
Permanently discontinue LUTATHERA for Grade 3 or higher toxicity requiring treatment delay of 16 weeks or longer.
Recurrent Grade 3 or 4
Permanently discontinue LUTATHERA.
2.5 Preparation and Administration
- Use aseptic technique and radiation shielding when administering the LUTATHERA solution. Use tongs when handling vial to minimize radiation exposure.
- Do not inject LUTATHERA directly into any other intravenous solution.
- Confirm the amount of radioactivity of LUTATHERA in the radiopharmaceutical vial with an appropriate dose calibrator prior to and after LUTATHERA administration.
- Inspect the product visually for particulate matter and discoloration prior to administration under a shielded screen. Discard vial if particulates or discoloration are present.
Administration Instructions
- Insert a 2.5 cm, 20 gauge needle (short needle) into the LUTATHERA vial and connect via a catheter to 500 mL 0.9% sterile sodium chloride solution (used to transport LUTATHERA during the infusion). Ensure that the short needle does not touch the LUTATHERA solution in the vial and do not connect this short needle directly to the patient. Do not allow sodium chloride solution to flow into the LUTATHERA vial prior to the initiation of the LUTATHERA infusion and do not inject LUTATHERA directly into the sodium chloride solution.
- Insert a second needle that is 9 cm, 18 gauge (long needle) into the LUTATHERA vial ensuring that this long needle touches and is secured to the bottom of the LUTATHERA vial during the entire infusion. Connect the long needle to the patient by an intravenous catheter that is prefilled with 0.9% sterile sodium chloride and that is used exclusively for the LUTATHERA infusion into the patient.
- Use a clamp or pump to regulate the flow of the sodium chloride solution via the short needle into the LUTATHERA vial at a rate of 50 mL/hour to 100 mL/hour for 5 to 10 minutes and then 200 mL/hour to 300 mL/hour for an additional 25 to 30 minutes (the sodium chloride solution entering the vial through the short needle will carry the LUTATHERA from the vial to the patient via the catheter connected to the long needle over a total duration of 30 to 40 minutes).
- Do not administer LUTATHERA as an intravenous bolus.
- During the infusion, ensure that the level of solution in the LUTATHERA vial remains constant
- Disconnect the vial from the long needle line and clamp the saline line once the level of radioactivity is stable for at least five minutes.
- Follow the infusion with an intravenous flush of 25 mL of 0.9% sterile sodium chloride.
- Dispose of any unused medicinal product or waste material in accordance with local and federal laws.
2.6 Radiation Dosimetry
The mean and standard deviation (SD) of the estimated radiation absorbed doses for adults receiving LUTATHERA are shown in Table 3. The maximum penetration in tissue is 2.2 mm and the mean penetration is 0.67 mm.
Table 3. Estimated Radiation Absorbed Dose for LUTATHERA in NETTER-1 - * N=18 (two patients excluded because the liver absorbed dose was biased by the uptake of the liver metastases)
- † N=9 (female patients only)
- ‡ N=11 (male patients only)
Absorbed dose per unit activity
(Gy/GBq)
(N=20)
Calculated absorbed dose for 4 x 7.4 GBq
(29.6 GBq cumulative activity)
(Gy)
Organ
Mean
SD
Mean
SD
Adrenals
0.037
0.016
1.1
0.5
Brain
0.027
0.016
0.8
0.5
Breasts
0.027
0.015
0.8
0.4
Gallbladder Wall
0.042
0.019
1.2
0.6
Heart Wall
0.032
0.015
0.9
0.4
Kidneys
0.654
0.295
19.4
8.7
Liver *
0.299
0.226
8.9
6.7
Lower Large Intestine Wall
0.029
0.016
0.9
0.5
Lungs
0.031
0.015
0.9
0.4
Muscle
0.029
0.015
0.8
0.4
Osteogenic Cells
0.151
0.268
4.5
7.9
Ovaries †
0.031
0.013
0.9
0.4
Pancreas
0.038
0.016
1.1
0.5
Red Marrow
0.035
0.029
1.0
0.8
Skin
0.027
0.015
0.8
0.4
Small Intestine
0.031
0.015
0.9
0.5
Spleen
0.846
0.804
25.1
23.8
Stomach Wall
0.032
0.015
0.9
0.5
Testes ‡
0.026
0.018
0.8
0.5
Thymus
0.028
0.015
0.8
0.5
Thyroid
0.027
0.016
0.8
0.5
Total Body
0.052
0.027
1.6
0.8
Upper Large Intestine Wall
0.032
0.015
0.9
0.4
Urinary Bladder Wall
0.437
0.176
12.8
5.3
Uterus
0.032
0.013
1.0
0.4
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk from Radiation Exposure
LUTATHERA contributes to a patient’s overall long-term radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer.
Radiation can be detected in the urine for up to 30 days following LUTATHERA administration. Minimize radiation exposure to patients, medical personnel, and household contacts during and after treatment with LUTATHERA consistent with institutional good radiation safety practices and patient management procedures [see Dosage and Administration ( 2.1)] .
5.2 Myelosuppression
In NETTER-1, myelosuppression occurred more frequently in patients receiving LUTATHERA with long-acting octreotide compared to patients receiving high-dose long-acting octreotide (all grades/grade 3 or 4): anemia (81%/0) versus (54%/1%); thrombocytopenia (53%/1%) versus (17%/0); and neutropenia (26%/3%) versus (11%/0). In NETTER-1, platelet nadir occurred at a median of 5.1 weeks following the first dose. Of the 59 patients who developed thrombocytopenia, 68% had platelet recovery to baseline or normal levels. The median time to platelet recovery was 2 months. Fifteen of the nineteen patients in whom platelet recovery was not documented had post-nadir platelet counts. Among these 15 patients, 5 improved to Grade 1, 9 to Grade 2, and 1 to Grade 3.
Monitor blood cell counts. Withhold, reduce dose, or permanently discontinue based on severity of adverse reaction [see Dosage and Administration ( 2.4)] .
5.3 Secondary Myelodysplastic Syndrome and Leukemia
In NETTER-1, with a median follow-up time of 24 months, myelodysplastic syndrome (MDS) was reported in 2.7% of patients receiving LUTATHERA with long-acting octreotide compared to no patients receiving high-dose long-acting octreotide. In ERASMUS, 15 patients (1.8%) developed MDS and 4 (0.5%) developed acute leukemia. The median time to the development of MDS was 28 months (9 to 41 months) for MDS and 55 months (32 to 155 months) for acute leukemia.
5.4 Renal Toxicity
In ERASMUS, 8 patients (<1%) developed renal failure 3 to 36 months following LUTATHERA. Two of these patients had underlying renal impairment or risk factors for renal failure (e.g., diabetes or hypertension) and required dialysis.
Administer the recommended amino acid solution before, during and after LUTATHERA [see Dosage and Administration ( 2.3)] to decrease reabsorption of lutetium Lu 177 dotatate through the proximal tubules and decrease the radiation dose to the kidneys. Do not decrease the dose of the amino acid solution if the dose of LUTATHERA is reduced. Advise patients to urinate frequently during and after administration of LUTATHERA. Monitor serum creatinine and calculated creatinine clearance. Withhold, reduce dose, or permanently discontinue LUTATHERA based on severity of reaction [see Dosage and Administration ( 2.4)] .
Patients with baseline renal impairment may be at greater risk of toxicity; perform more frequent assessments of renal function in patients with mild or moderate impairment. LUTATHERA has not been studied in patients with severe renal impairment (creatinine clearance < 30 mL/min).
5.5 Hepatotoxicity
In ERASMUS, 2 patients (<1%) were reported to have hepatic tumor hemorrhage, edema, or necrosis, with one patient experiencing intrahepatic congestion and cholestasis. Patients with hepatic metastasis may be at increased risk of hepatotoxicity due to radiation exposure.
Monitor transaminases, bilirubin and serum albumin during treatment. Withhold, reduce dose, or permanently discontinue LUTATHERA based on severity of reaction [see Dosage and Administration ( 2.2)] .
5.6 Neuroendocrine Hormonal Crisis
Neuroendocrine hormonal crises, manifesting with flushing, diarrhea, bronchospasm and hypotension, occurred in 1% of patients in ERASMUS and typically occurred during or within 24 hours following the initial LUTATHERA dose. Two (<1%) patients were reported to have hypercalcemia.
Monitor patients for flushing, diarrhea, hypotension, bronchoconstriction or other signs and symptoms of tumor-related hormonal release. Administer intravenous somatostatin analogs, fluids, corticosteroids, and electrolytes as indicated.
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action, LUTATHERA can cause fetal harm [see Clinical Pharmacology ( 12.1)] . There are no available data on the use of LUTATHERA in pregnant women. No animal studies using lutetium Lu 177 dotatate have been conducted to evaluate its effect on female reproduction and embryo-fetal development; however, all radiopharmaceuticals, including LUTATHERA, have the potential to cause fetal harm.
Verify pregnancy status of females of reproductive potential prior to initiating LUTATHERA [see Dosage and Administration ( 2.1)] .
Advise females and males of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with LUTATHERA and for 7 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the final dose [see Use in Specific Populations ( 8.1, 8.3)] .
5.8 Risk of Infertility
LUTATHERA may cause infertility in males and females. The recommended cumulative dose of 29.6 GBq of LUTATHERA results in a radiation absorbed dose to the testis and ovaries within the range where temporary or permanent infertility can be expected following external beam radiotherapy [see Dosage and Administration ( 2.6) and Use in Specific Populations ( 8.3)] .
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling.
- Myelosuppression [see Warnings and Precautions ( 5.2)]
- Secondary Myelodysplastic Syndrome and Leukemia [see Warnings and Precautions ( 5.3)]
- Renal Toxicity [see Warnings and Precautions ( 5.4)]
- Hepatotoxicity [see Warnings and Precautions ( 5.5)]
- Neuroendocrine Hormonal Crisis [see Warnings and Precautions ( 5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in Warnings and Precautions reflect exposure to LUTATHERA in 111 patients with advanced, progressive midgut neuroendocrine tumors (NETTER-1). Safety data in Warnings and Precautions were also obtained in an additional 22 patients in a non-randomized pharmacokinetic substudy of NETTER-1 and in a subset of patients (811 of 1214) with advanced somatostatin receptor-positive tumors enrolled in ERASMUS [see Warnings and Precautions ( 5)] .
NETTER-1
The safety data described below are from NETTER-1, which randomized (1:1) patients with progressive, somatostatin receptor-positive midgut carcinoid tumors to receive LUTATHERA 7.4 GBq (200 mCi) administered every 8 to 16 weeks concurrently with the recommended amino acid solution and with long-acting octreotide (30 mg administered by intramuscular injection within 24 hours of each LUTATHERA dose) (n = 111), or high-dose octreotide (defined as long-acting octreotide 60 mg by intramuscular injection every 4 weeks) (n = 112) [see Clinical Studies ( 14.1)] . Among patients receiving LUTATHERA with octreotide, 79% received a cumulative dose > 22.2 GBq (> 600 mCi) and 76% of patients received all four planned doses. Six percent (6%) of patients required a dose reduction and 13% of patients discontinued LUTATHERA. Five patients discontinued LUTATHERA for renal-related events and 4 discontinued for hematological toxicities. The median duration of follow-up was 24 months for patients receiving LUTATHERA with octreotide and 20 months for patients receiving high-dose octreotide.Table 4 and Table 5 summarize the incidence of adverse reactions and laboratory abnormalities, respectively. The most common Grade 3-4 adverse reactions occurring with a greater frequency among patients receiving LUTATHERA with octreotide compared to patients receiving high-dose octreotide include: lymphopenia (44%), increased GGT (20%), vomiting (7%), nausea and elevated AST (5% each), and increased ALT, hyperglycemia and hypokalemia (4% each).
Table 4. Adverse Reactions Occurring in ≥ 5% (All Grades) of Patients Receiving LUTATHERA with Octreotide in NETTER-1 * - * National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Only displays adverse reactions occurring at a higher incidence in LUTATHERA-treated patients [between arm difference of ≥5% (all grades) or ≥2% (grades 3-4)]
- † Includes the terms: Glomerular filtration rate decreased, acute kidney injury, acute prerenal failure, azotemia, renal disorder, renal failure, renal impairment
- ‡ Includes the terms: Dysuria, micturition urgency, nocturia, pollakiuria, renal colic, renal pain, urinary tract pain and urinary incontinence
Adverse Reaction *
LUTATHERA and Long-Acting Octreotide (30 mg) (N = 111)
Long-Acting Octreotide (60 mg)
(N = 112)
All Grades %
Grades 3-4 %
All Grades %
Grades 3-4 %
Cardiac disorders
Atrial fibrillation
5
1
0
0
Gastrointestinal disorders
Nausea
65
5
12
2
Vomiting
53
7
9
0
Abdominal pain
26
3
19
3
Diarrhea
26
3
18
1
Constipation
10
0
5
0
General disorders
Fatigue
38
1
26
2
Peripheral edema
16
0
9
1
Pyrexia
8
0
3
0
Metabolism and nutrition disorders
Decreased appetite
21
0
11
3
Musculoskeletal and connective tissue disorders
Back pain
13
2
10
0
Pain in extremity
11
0
5
0
Myalgia
5
0
0
0
Neck Pain
5
0
0
0
Nervous system disorders
Headache
17
0
5
0
Dizziness
17
0
8
0
Dysgeusia
8
0
2
0
Psychiatric disorders
Anxiety
12
1
5
0
Renal and urinary disorders
Renal failure †
12
3
3
1
Radiation-related urinary tract toxicity ‡
8
0
3
0
Respiratory, thoracic and mediastinal disorders
Cough
11
1
6
0
Skin and subcutaneous tissue disorders
Alopecia
12
0
2
0
Vascular disorders
Flushing
14
1
9
0
Hypertension
12
2
7
2
Table 5. Laboratory Abnormalities Occurring in ≥ 5% (All Grades) of Patients Receiving LUTATHERA with Octreotide in NETTER-1 *† - * Values are worst grade observed after randomization
- † National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Only displays laboratory abnormalities occurring at a higher incidence in LUTATHERA-treated patients [between arm difference of ≥5% (all grades) or ≥2%(grades 3-4)]
Laboratory Abnormality †
LUTATHERA and Long-Acting
Octreotide (30 mg) (N = 111)
Long-Acting Octreotide (60 mg)
(N = 112)All grades %
Grade 3-4 %
All grades %
Grade 3-4 %
Hematology
Lymphopenia
90
44
39
4
Anemia
81
0
54
1
Leukopenia
55
2
20
0
Thrombocytopenia
53
1
17
0
Neutropenia
26
3
11
0
Renal/Metabolic
Creatinine increased
85
1
73
0
Hyperglycemia
82
4
67
2
Hyperuricemia
34
6
29
6
Hypocalcemia
32
0
14
0
Hypokalemia
26
4
21
2
Hyperkalemia
19
0
11
0
Hypernatremia
17
0
7
0
Hypoglycemia
15
0
8
0
Hepatic
GGT increased
66
20
67
16
Alkaline phosphatase increased
65
5
54
9
AST increased
50
5
35
0
ALT increased
43
4
34
0
Blood bilirubin increased
30
2
28
0
ERASMUS
Safety data are available from 1214 patients in ERASMUS, an international, single-institution, single-arm, open-label trial of patients with somatostatin receptor-positive tumors (neuroendocrine and other primaries). Patients received LUTATHERA 7.4 GBq (200 mCi) administered every 6 to 13 weeks with or without octreotide. Retrospective medical record review was conducted on a subset of 811 patients to document serious adverse reactions. Eighty-one (81%) percent of patients in the subset received a cumulative dose ≥ 22.2 GBq (≥ 600 mCi). With a median follow-up time of more than 4 years, the following rates of serious adverse reactions were reported: myelodysplastic syndrome (2%), acute leukemia (1%), renal failure (2%), hypotension (1%), cardiac failure (2%), myocardial infarction (1%), and neuroendocrine hormonal crisis (1%). -
7 DRUG INTERACTIONS
7.1 Somatostatin Analogs
Somatostatin and its analogs competitively bind to somatostatin receptors and may interfere with the efficacy of LUTATHERA. Discontinue long-acting somatostatin analogs at least 4 weeks and short-acting octreotide at least 24 hours prior to each LUTATHERA dose. Administer short- and long-acting octreotide during LUTATHERA treatment as recommended [see Dosage and Administration ( 2.3)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, LUTATHERA can cause fetal harm [see Clinical Pharmacology ( 12.1)] . There are no available data on LUTATHERA use in pregnant women. No animal studies using lutetium Lu 177 dotatate have been conducted to evaluate its effect on female reproduction and embryo-fetal development; however, all radiopharmaceuticals, including LUTATHERA, have the potential to cause fetal harm. Advise pregnant women of the risk to a fetus.In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of lutetium Lu 177 dotatate in human milk, or its effects on the breastfed infant or milk production. No lactation studies in animals were conducted. Because of the potential risk for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment with LUTATHERA and for 2.5 months after the final dose.8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating LUTATHERA [see Use in Specific Populations ( 8.1)] .Contraception
Females
LUTATHERA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)] . Advise females of reproductive potential to use effective contraception during treatment and for 7 months following the final dose of LUTATHERA.Males
Based on its mechanism of action, advise males with female partners of reproductive potential to use effective contraception during and for 4 months following the final dose of LUTATHERA [see Clinical Pharmacology ( 12.1) and Nonclinical Toxicology ( 13.1)] .Infertility
The recommended cumulative dose of 29.6 GBq of LUTATHERA results in a radiation absorbed dose to the testis and ovaries within the range where temporary or permanent infertility can be expected following external beam radiotherapy [see Dosage and Administration ( 2.6)] .8.4 Pediatric Use
The safety and effectiveness of LUTATHERA have not been established in pediatric patients.
8.5 Geriatric Use
Of the 1325 patients treated with LUTATHERA in clinical trials, 438 patients (33%) were 65 years and older. The response rate and number of patients with a serious adverse event were similar to that of younger subjects.
8.6 Renal Impairment
No dose adjustment is recommended for patients with mild to moderate renal impairment; however, patients with mild or moderate renal impairment may be at greater risk of toxicity. Perform more frequent assessments of renal function in patients with mild to moderate impairment. The safety of LUTATHERA in patients with severe renal impairment (creatinine clearance < 30 mL/min by Cockcroft-Gault) or end-stage renal disease has not been studied.
-
11 DESCRIPTION
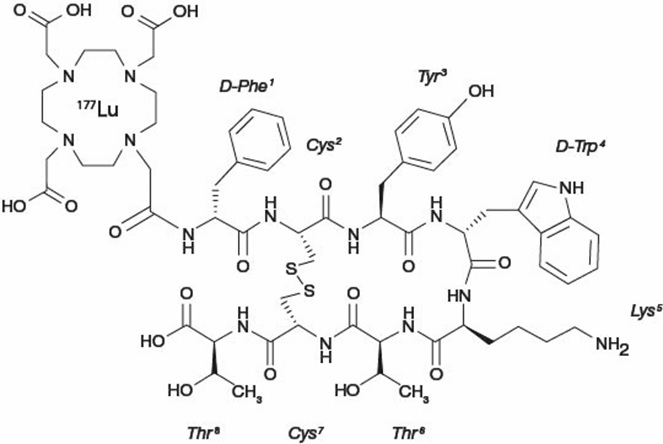
LUTATHERA (lutetium Lu 177 dotatate) is a radiolabeled somatostatin analog. The drug substance lutetium Lu 177 dotatate is a cyclic peptide linked with the covalently bound chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid to a radionuclide.
Lutetium Lu 177 dotatate is described as lutetium (Lu 177)-N-[(4,7,10-Tricarboxymethyl-1,4,7,10-tetraazacyclododec-1-yl) acetyl]-D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophanyl-L-lysyl-L-threoninyl-L-cysteinyl-L-threonine-cyclic (2-7) disulfide. The molecular weight is 1609.6 Daltons and the structural formula is as follows:
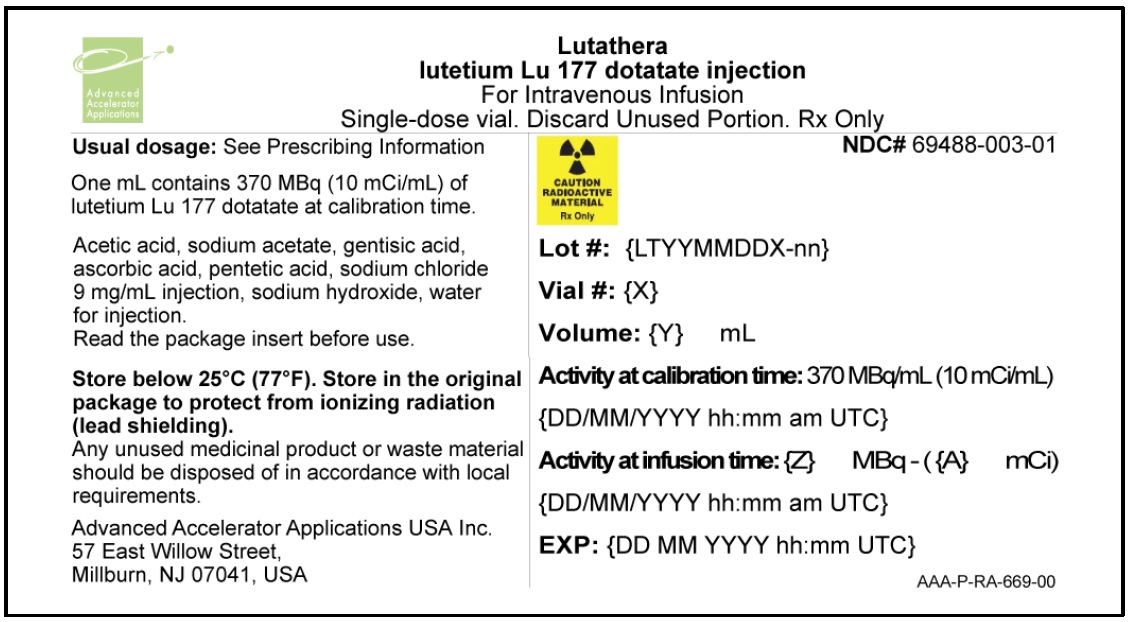
LUTATHERA (lutetium Lu 177 dotatate) 370 MBq/mL (10 mCi/mL) Injection is a sterile, clear, colorless to slightly yellow solution for intravenous use. Each single-dose vial contains acetic acid (0.48 mg/mL), sodium acetate (0.66 mg/mL), gentisic acid (0.63 mg/mL), sodium hydroxide (0.65 mg/mL), ascorbic acid (2.8 mg/mL), diethylene triamine pentaacetic acid (0.05 mg/mL), sodium chloride (6.85 mg/mL), and Water for Injection (ad 1 mL). The pH range of the solution is 4.5 to 6.
11.1 Physical Characteristics
Lutetium (Lu 177) decays to stable hafnium (Hf 177) with a half-life of 6.647 days, by emitting beta radiation with a maximum energy of 0.498 MeV and photonic radiation (γ) of 0.208 MeV (11%) and 0.113 MeV (6.4%). The main radiations are detailed in Table 6.
Table 6. Lu 177 Main Radiations Radiation
Energy (keV)
Iβ%
Iγ%
β -
176.5
12.2
β -
248.1
0.05
β -
384.9
9.1
β -
497.8
78.6
γ
71.6
0.15
γ
112.9
6.40
γ
136.7
0.05
γ
208.4
11.0
γ
249.7
0.21
γ
321.3
0.22
11.2 External Radiation
Table 7 summarizes the radioactive decay properties of Lu 177.
Table 7. Physical Decay Chart: Lutetium Lu 177 Half-life = 6.647 days Hours
Fraction Remaining
Hours
Fraction Remaining
0
1.000
48 (2 days)
0.812
1
0.996
72 (3 days)
0.731
2
0.991
168 (7 days)
0.482
5
0.979
336 (14 days)
0.232
10
0.958
720 (30 days)
0.044
24 (1 day)
0.901
1080 (45 days)
0.009
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lutetium Lu 177 dotatate binds to somatostatin receptors with highest affinity for subtype 2 receptors (SSRT2). Upon binding to somatostatin receptor expressing cells, including malignant somatostatin receptor-positive tumors, the compound is internalized. The beta emission from Lu 177 induces cellular damage by formation of free radicals in somatostatin receptor-positive cells and in neighboring cells.
12.2 Pharmacodynamics
Lutetium Lu 177 exposure-response relationships and the time course of pharmacodynamics response are unknown.
Cardiac Electrophysiology
The ability of LUTATHERA to prolong the QTc interval at the therapeutic dose was assessed in an open label study in 20 patients with somatostatin receptor-positive midgut carcinoid tumors. No large changes in the mean QTc interval (i.e., >20 ms) were detected.12.3 Pharmacokinetics
The pharmacokinetics (PK) of lutetium Lu 177 dotatate have been characterized in patients with progressive, somatostatin receptor-positive neuroendocrine tumors. The mean blood exposure (AUC) of lutetium Lu 177 dotatate at the recommended dose is 41 ng.h/mL [coefficient of variation (CV) 36 %]. The mean maximum blood concentration (C max) for lutetium Lu 177 dotatate is 10 ng/mL (CV 50%), which generally occurred at the end of the LUTATHERA infusion.
Distribution
The mean volume of distribution for lutetium Lu 177 dotatate is 460 L (CV 54%).Within 4 hours after administration, lutetium Lu 177 dotatate distributes in kidneys, tumor lesions, liver, spleen, and, in some patients, pituitary gland and thyroid. The co-administration of amino acids reduced the median radiation dose to the kidneys by 47% (34% to 59%) and increased the mean beta-phase blood clearance of lutetium Lu 177 dotatate by 36%.
The non-radioactive form of lutetium dotatate is 43% bound to human plasma proteins.
Elimination
The mean clearance (CL) is 4.5 L/h (CV 31%) for lutetium Lu 177 dotatate. The mean (± standard deviation) effective blood elimination half-life is 3.5 (±1.4) hours and the mean terminal blood half-life is 71 (± 28) hours.Metabolism
Lutetium Lu 177 dotatate does not undergo hepatic metabolism.Excretion
Lutetium Lu 177 dotatate is primarily eliminated renally with cumulative excretion of 44% within 5 hours, 58% within 24 hours, and 65% within 48 hours following LUTATHERA administration. Prolonged elimination of lutetium Lu 177 dotatate in the urine is expected; however, based on the half-life of lutetium 177 and terminal half-life of lutetium Lu 177 dotatate, greater than 99% will be eliminated within 14 days after administration of LUTATHERA [see Warnings and Precautions ( 5.1)] .Drug Interaction Studies
The non-radioactive form of lutetium is not an inhibitor or inducer of cytochrome P450 (CYP) 1A2, 2B6, 2C9, 2C19 or 2D6 in vitro. It is not an inhibitor of P-glycoprotein, BCRP, OAT1, OAT3, OCT2, OATP1B1, OATP1B3, or OCT1 in vitro. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with Lutetium Lu 177 dotatate; however, radiation is a carcinogen and mutagen.
No animal studies were conducted to determine the effects of lutetium Lu 177 dotatate on fertility.
13.2 Animal Toxicology and/or Pharmacology
The primary target organ in animal studies using a non-radioactive form of lutetium Lu 177 dotatate (lutetium Lu 175 dotatate) was the pancreas, a high SSTR2 expressing organ. Pancreatic acinar apoptosis occurred at lutetium Lu 175 dotatate doses ≥ 5 mg/kg in repeat dose toxicology studies in rats. Pancreatic acinar cell atrophy also occurred in repeat dose toxicology studies in dogs at doses ≥ 500 mg/kg. These findings were consistent with high uptake of the radiolabeled peptide in the pancreas in animal biodistribution studies.
-
14 CLINICAL STUDIES
14.1 Progressive, Well-differentiated Advanced or Metastatic Somatostatin Receptor-Positive Midgut Carcinoid Tumors
The efficacy of LUTATHERA in patients with progressive, well-differentiated, locally advanced/inoperable or metastatic somatostatin receptor-positive midgut carcinoid tumors was established in NETTER-1 (NCT01578239), a randomized, multicenter, open-label, active-controlled trial. Key eligibility criteria included Ki67 index ≤ 20%, Karnofsky performance status ≥ 60, confirmed presence of somatostatin receptors on all lesions (OctreoScan uptake ≥ normal liver), creatinine clearance ≥ 50 mL/min, no prior treatment with peptide receptor radionuclide therapy (PRRT), and no prior external radiation therapy to more than 25% of the bone marrow.
Two hundred twenty-nine (229) patients were randomized (1:1) to receive either LUTATHERA 7.4 GBq (200 mCi) every 8 weeks for up to 4 administrations (maximum cumulative dose of 29.6 GBq) or high-dose long-acting octreotide (defined as 60 mg by intramuscular injection every 4 weeks). Patients in the LUTATHERA arm also received long-acting octreotide 30 mg as an intramuscular injection 4 to 24 hours after each LUTATHERA dose and every 4 weeks after completion of LUTATHERA treatment until disease progression or until week 76 of the study. Patients in both arms could receive short-acting octreotide for symptom management; however, short-acting octreotide was withheld for at least 24 hours before each LUTATHERA dose. Randomization was stratified by OctreoScan tumor uptake score (Grade 2, 3 or 4) and the length of time that patients had been on the most recent constant dose of octreotide prior to randomization (≤ 6 or > 6 months). The major efficacy outcome measure was progression free survival (PFS) as determined by a blinded independent radiology committee (IRC) per RECIST v1.1. Additional efficacy outcome measures were overall response rate (ORR) by IRC, duration of response, and overall survival (OS).
Demographic and baseline disease characteristics were balanced between the treatment arms. Of the 208 patients, whose race/ethnicity was reported, 90% were White, 5% were Black, and 4% were Hispanic or Latino. The median age was 64 years (28 to 87 years); 51% were male, 74% had an illial primary, and 96% had metastatic disease in the liver. The median Karnofsky performance score was 90 (60 to 100), 74% received a constant dose of octreotide for > 6 months and 12% received prior treatment with everolimus. Sixty-nine percent of patients had Ki67 expression in ≤ 2% of tumor cells, 77% had CgA > 2 times the upper limit of normal (ULN), 65% had 5-HIAA > 2 x ULN, and 65% had alkaline phosphatase ≤ ULN. Efficacy results for NETTER-1 are presented in Table 8 and Figure 1.
Table 8. Efficacy Results in NETTER-1 - * Median follow-up 10.5 months at time of primary analysis of PFS (range: 0 to 29 months)
- † Hazard ratio based on the unstratified Cox model
- ‡ Unstratified log rank test
- § Interim analysis of OS not statistically significant based on pre-specified significance criteria
- ¶ Fisher’s Exact test
LUTATHERA and Long-Acting Octreotide (30 mg)
N=116Long-Acting Octreotide (60 mg)
N=113PFS by IRC
Events (%)
27 (23%)
78 (69%)
Progressive disease, n (%)
15 (13%)
61 (54%)
Death, n (%)
12 (10%)
17 (15%)
Median in months (95% CI)
NR * (NE, NE)
8.5 (5.8, 9.1)
Hazard ratio † (95% CI)
0.21 (0.13, 0.32)
P-Value ‡
< 0.0001
OS (Updated)
Deaths (%)
27 (23%)
43 (38%)
Median in months (95% CI)
NR (31.0, NE)
27.4 (22.2, NE)
0.52 (0.32, 0.84)
ORR by IRC
ORR, % (95% CI)
13% (7%,19%)
4% (0.1%, 7%)
Complete response rate, n (%)
1 (1%)
0
Partial response rate, n (%)
14 (12%)
4 (4%)
P-Value ¶
0.0148
Duration of response, median in months (95% CI)
NR (2.8, NE)
1.9 (1.9, NE)
NR: Not reached;
NE: Not evaluableFigure 1. Kaplan-Meier Curves for Progression-Free Survival in NETTER-1
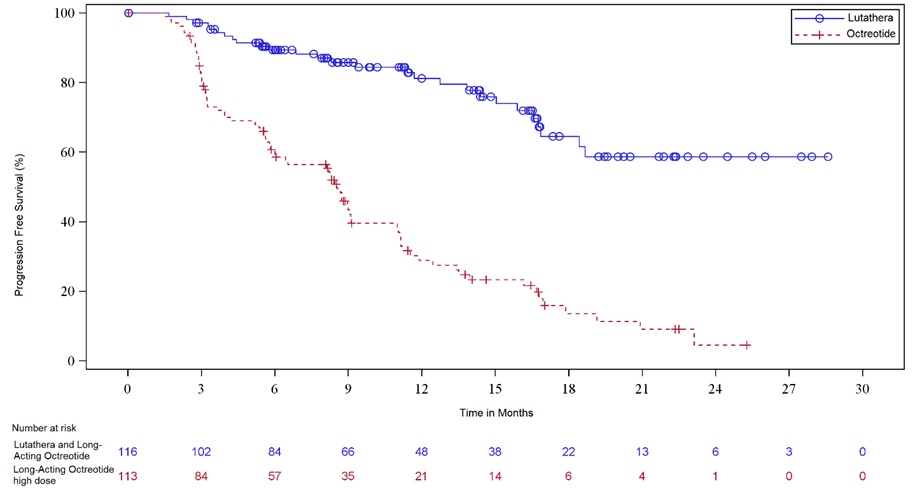
14.2 Somatostatin Receptor-Positive Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs)
The efficacy of LUTATHERA in patients with foregut, midgut, and hindgut gastroenteropancreatic neuroendocrine tumors (GEP-NETs) was assessed in 360 patients in the ERASMUS study. In ERASMUS, LUTATHERA was initially provided as expanded access under a general peptide receptor radionuclide therapy protocol at a single site in the Netherlands. A subsequent LUTATHERA-specific protocol written eight years after study initiation did not describe a specific sample size or hypothesis testing plan but allowed for retrospective data collection. A total of 1214 patients received LUTATHERA in ERASMUS, of which 601 (50%) were assessed per RECIST criteria. Of the 601 patients evaluated by investigators using RECIST criteria, 360 (60%) had gastroentero-pancreatic neuroendocrine tumors (GEP-NETs). LUTATHERA 7.4 GBq (200 mCi) was administered every 6 to 13 weeks for up to 4 doses concurrently with the recommended amino acid solution. The major efficacy outcome was investigator-assessed ORR. The median age in the efficacy subset was 61 years (25 to 88 years), 52% were male, 61% had a baseline Karnofsky performance status ≥ 90 (60 to 100), 60% had progressed within 12 months of treatment, and 15% had received prior chemotherapy. Fifty five percent (55%) of patients received a concomitant somatostatin analog. The median dose of LUTATHERA was 29.6 GBq (800 mCi). Baseline tumor assessments were obtained in 39% of patients. The investigator assessed ORR was 16% (95% CI 13, 20) in the 360 patients with GEP-NETs. Three complete responses were observed (< 1%). Median DoR in the 58 responding patients was 35 months (95% CI: 17, 38).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
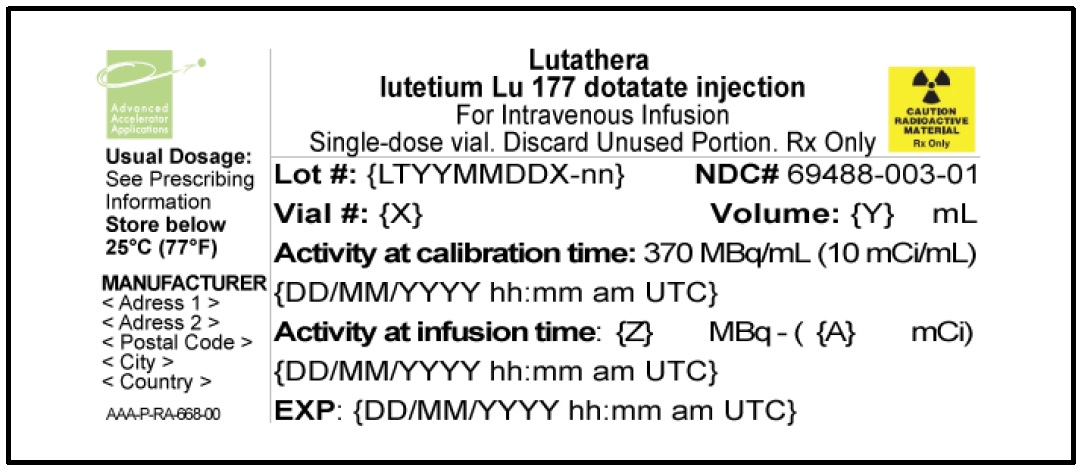
LUTATHERA Injection containing 370 MBq/mL (10 mCi/ml) of lutetium Lu 177 dotatate is a sterile, preservative-free and clear, colorless to slightly yellow solution for intravenous use supplied in a colorless Type I glass 30 mL single-dose vial containing 7.4 GBq (200 mCi) ± 10% of lutetium Lu 177 dotatate at the time of injection ( NDC# 69488-003-01). The solution volume in the vial is adjusted from 20.5 mL to 25 mL to provide a total of 7.4 GBq (200 mCi) of radioactivity.
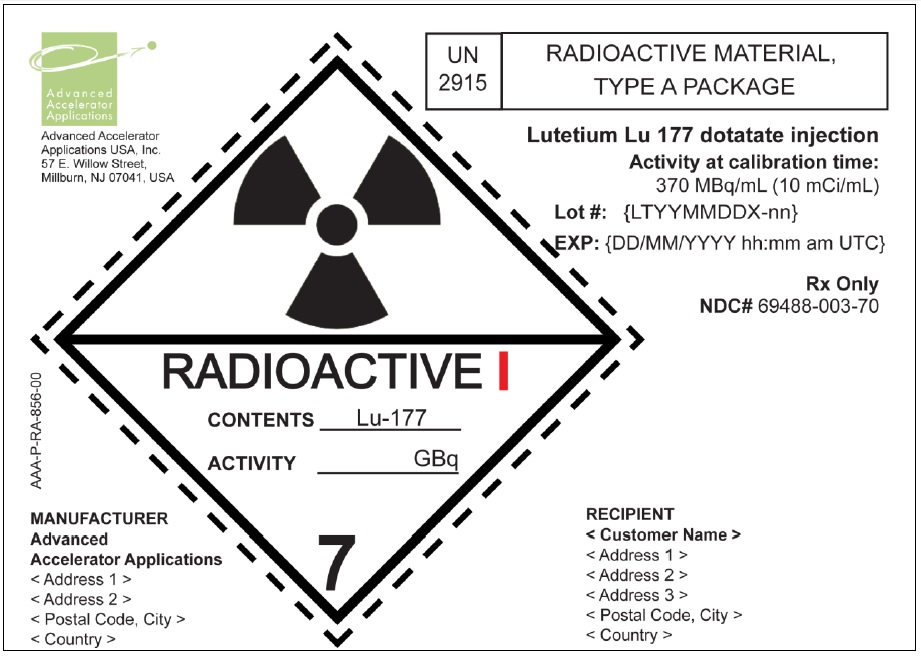
The product vial is in a lead shielded container placed in a plastic sealed container ( NDC# 69488-003-01). The product is shipped in a Type A package ( NDC# 69488-003-70).
Store below 25 °C (77 °F).
The shelf life is 72 hours. Discard appropriately at 72 hours.
-
17 PATIENT COUNSELING INFORMATION
Radiation Risks
Advise patients to minimize radiation exposure to household contacts consistent with institutional good radiation safety practices and patient management procedures [see Dosage and Administration ( 2.1), Warnings and Precautions ( 5.1)] .Myelosuppression
Advise patients to contact their healthcare provider for any signs or symptoms of myelosuppression or infection, such as fever, chills, dizziness, shortness of breath, or increased bleeding or bruising [see Warnings and Precautions ( 5.2)] .Secondary Myelodysplastic Syndrome and Acute Leukemia
Advise patients of the potential for secondary cancers, including myelodysplastic syndrome and acute leukemia [see Warnings and Precautions ( 5.3)] .Renal Toxicity
Advise patients to hydrate and urinate frequently during and after administration of LUTATHERA [see Warnings and Precautions ( 5.4)] .Hepatotoxicity
Advise patients of the need for periodic laboratory tests to monitor for hepatotoxicity [see Warnings and Precautions ( 5.5)] .Neuroendocrine Hormonal Crises
Advise patients to contact their health care provider for signs or symptoms that may occur following tumor-hormone release, including severe flushing, diarrhea, bronchospasm, and hypotension [see Warnings and Precautions ( 5.6)] .Embryo-Fetal Toxicity
Advise pregnant women and males and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions ( 5.7), Use in Specific Populations ( 8.1, 8.3)] .Advise females of reproductive potential to use effective contraception during treatment with LUTATHERA and for 7 months after the final dose [see Use in Specific Populations ( 8.1, 8.3)] .
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with LUTATHERA and for 4 months after the final dose [see Use in Specific Populations ( 8.1, 8.3)] .
Lactation
Advise females not to breastfeed during treatment with LUTATHERA and for 2.5 months after the final dose [see Use in Specific Populations ( 8.2)] .Infertility
Advise female and male patients that LUTATHERA may impair fertility [see Warnings and Precautions ( 5.8), Use in Specific Populations ( 8.3)] .Manufactured by:
Advanced Accelerator Applications, S.r.l.
Via Ribes 5, 10010 Colleretto Giacosa (TO), ItalyAdvanced Accelerator Applications, S.r.l.
Via Piero Maroncelli 40/1, 47014 Meldola (FC), ItalyOr
Advanced Accelerator Applications USA, Inc.
57 East Willow Street, Millburn, NJ 07041, USA
Distributed by:
Advanced Accelerator Applications USA, Inc., NJ 07041
© Advanced Accelerator Applications USA, Inc. 2018
LUTATHERA® is a registered trademark of Advanced Accelerator Applications S.A.
U.S. Patents 5830431; 5804157 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUTATHERA
lutetium lu 177 dotatate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69488-003 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LUTETIUM OXODOTREOTIDE LU-177 (UNII: AE221IM3BB) (LUTETIUM OXODOTREOTIDE LU-177 - UNII:AE221IM3BB) LUTETIUM OXODOTREOTIDE LU-177 10 mCi in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SODIUM ACETATE (UNII: 4550K0SC9B) GENTISIC ACID (UNII: VP36V95O3T) SODIUM HYDROXIDE (UNII: 55X04QC32I) PENTETIC ACID (UNII: 7A314HQM0I) SODIUM CHLORIDE (UNII: 451W47IQ8X) ASCORBIC ACID (UNII: PQ6CK8PD0R) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69488-003-01 1 in 1 PACKAGE 01/26/2018 1 20.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208700 01/26/2018 Labeler - Advanced Accelerator Applications USA, Inc (051714355) Establishment Name Address ID/FEI Business Operations Advanced Accelerator Applications USA, Inc. 080178357 manufacture(69488-003) Establishment Name Address ID/FEI Business Operations Advanced Accelerator Applications (Italy) srl 338664304 manufacture(69488-003) Establishment Name Address ID/FEI Business Operations Advanced Accelerator Applications (Italy) srl 428469971 manufacture(69488-003)
Trademark Results [Lutathera]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LUTATHERA 79101923 4196427 Live/Registered |
ADVANCED ACCELERATOR APPLICATIONS INTERN 2011-07-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.