VISCO SHIELD LUBRICANT (hypromellose 2208- 15000 mpa.s solution/ drops
VISCO SHIELD by
Drug Labeling and Warnings
VISCO SHIELD by is a Otc medication manufactured, distributed, or labeled by OASIS Medical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
-
Warnings
- For external use only
- To avoid contamination do not touch tip of container or applicator to any surface
- Once opened, discard
- Do not reuse
- Directions
- Questions, or comments
- Other information
- Inactive Ingredients
-
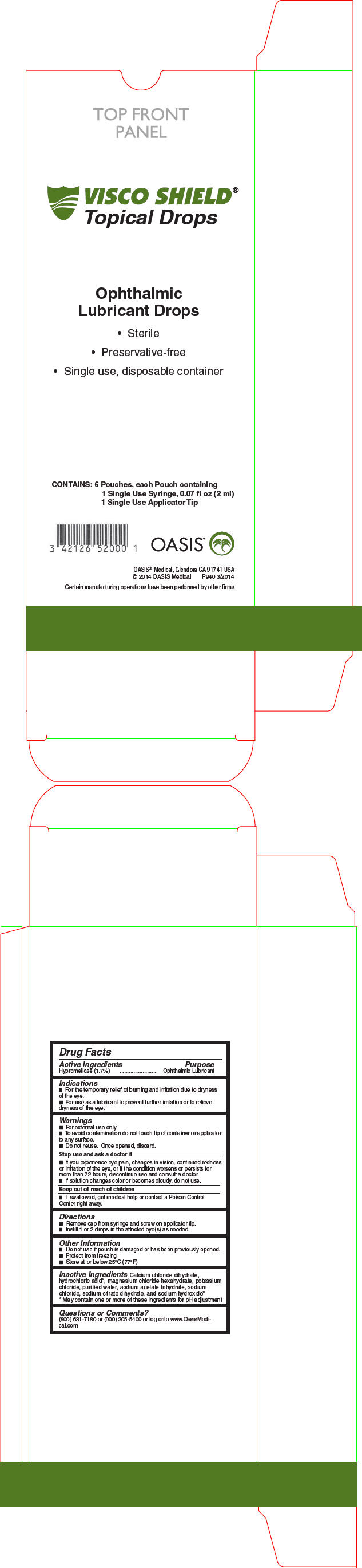
PRINCIPAL DISPLAY PANEL - 2 ml Syringe Carton
VISCO SHIELD®
Topical DropsOphthalmic
Lubricant Drops- Sterile
- Preservative-free
- Single use, disposable container
CONTAINS: 6 Pouches, each Pouch containing
1 Single Use Syringe, 0.07 fl oz (2 ml)
1 Single Use Applicator TipOASIS®
OASIS® Medical, Glendora CA 91741 USA
© 2014 OASIS Medical
P940 3/2014Certain manufacturing operations have been performed by other firms
-
INGREDIENTS AND APPEARANCE
VISCO SHIELD LUBRICANT
hypromellose 2208 (15000 mpa.s) solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42126-5200 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) (HYPROMELLOSE 2208 (15000 MPA.S) - UNII:Z78RG6M2N2) HYPROMELLOSE 2208 (15000 MPA.S) 17 mg in 1 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) HYDROCHLORIC ACID (UNII: QTT17582CB) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42126-5200-0 6 in 1 CARTON 06/16/2014 1 1 in 1 POUCH 1 2 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part349 06/16/2014 Labeler - OASIS Medical, Inc. (194121018) Establishment Name Address ID/FEI Business Operations OASIS Medical, Inc. 194121018 MANUFACTURE(42126-5200)
Trademark Results [VISCO SHIELD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VISCO SHIELD 75337185 2300472 Live/Registered |
Oasis Medical, Inc. 1997-08-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.