OLOPATADINE HYDROCHLORIDE solution/ drops
Olopatadine Hydrochloride by
Drug Labeling and Warnings
Olopatadine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Teva Pharmaceuticals USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OLOPATADINE HYDROCHLORIDE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for OLOPATADINE HYDROCHLORIDE OPHTHALMIC SOLUTION.
OLOPATADINE HYDROCHLORIDE ophthalmic solution 0.2%
Initial U.S. Approval: 1996INDICATIONS AND USAGE
Olopatadine hydrochloride ophthalmic solution is a mast cell stabilizer indicated for the treatment of ocular itching associated with allergic conjunctivitis. (1)
DOSAGE AND ADMINISTRATION
The recommended dose is one drop in each affected eye once a day. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution 0.2%: each mL contains 2.22 mg of olopatadine hydrochloride. (3)
WARNINGS AND PRECAUTIONS
For topical ocular use only. Not for injection or oral use. (5.1)
ADVERSE REACTIONS
Symptoms similar to cold syndrome and pharyngitis were reported at an incidence of approximately 10%. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-866-832-8537 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 For Topical Ocular Use Only
5.2 Contamination of Tip and Solution
5.3 Contact Lens Use
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Topical Ophthalmic Use Only
17.2 Sterility of Dropper Tip
17.3 Concomitant Use of Contact Lenses
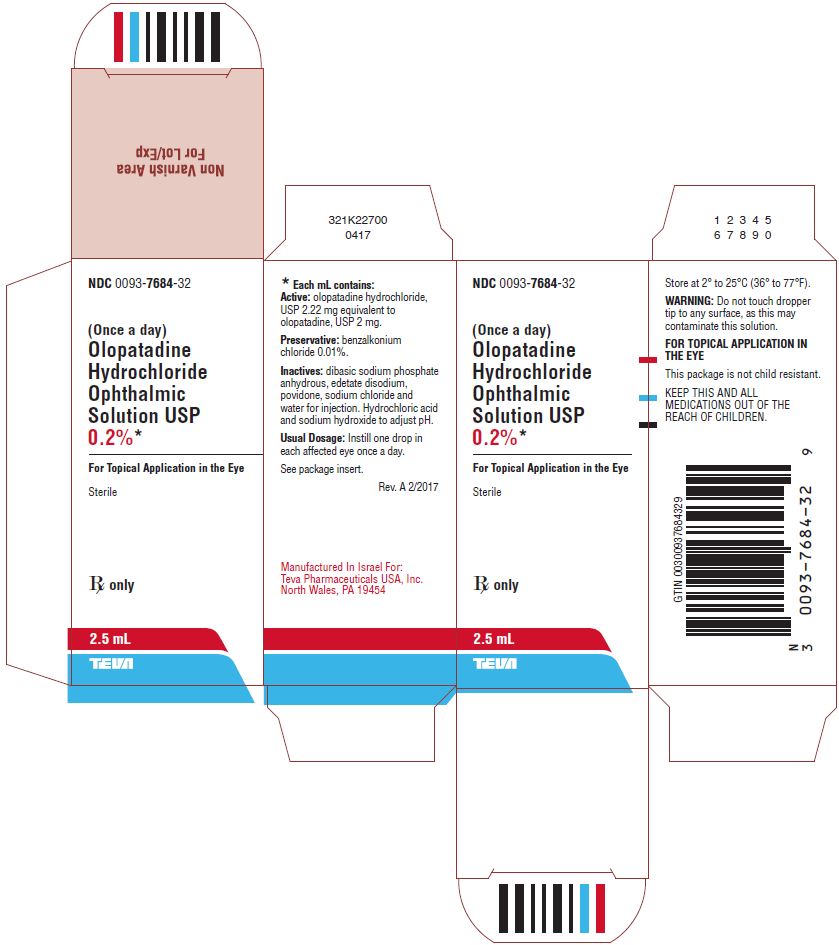
Olopatadine Hydrochloride Ophthalmic Solution USP 0.2%, 2.5 mL Bottle, Carton Text
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.2 Contamination of Tip and Solution
As with any eye drop, to prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
5.3 Contact Lens Use
Patients should be advised not to wear a contact lens if their eye is red.
Olopatadine hydrochloride ophthalmic solution 0.2% should not be used to treat contact lens related irritation.
The preservative in olopatadine hydrochloride ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red, should be instructed to wait at least ten minutes after instilling olopatadine hydrochloride ophthalmic solution 0.2% before they insert their contact lenses.
-
6 ADVERSE REACTIONS
Symptoms similar to cold syndrome and pharyngitis were reported at an incidence of approximately 10%.
The following adverse experiences have been reported in 5% or less of patients:
Ocular: blurred vision, burning or stinging, conjunctivitis, dry eye, foreign body sensation, hyperemia, hypersensitivity, keratitis, lid edema, pain and ocular pruritus.
Non-ocular: asthenia, back pain, flu syndrome, headache, increased cough, infection, nausea, rhinitis, sinusitis and taste perversion.
Some of these events were similar to the underlying disease being studied.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C
Olopatadine was found not to be teratogenic in rats and rabbits. However, rats treated at 600 mg/kg/day, or 150,000 times the MROHD and rabbits treated at 400 mg/kg/day, or approximately 100,000 times the MROHD, during organogenesis showed a decrease in live fetuses. In addition, rats treated with 600 mg/kg/day of olopatadine during organogenesis showed a decrease in fetal weight. Further, rats treated with 600 mg/kg/day of olopatadine during late gestation through the lactation period showed a decrease in neonatal survival and body weight. There are, however, no adequate and well- controlled studies in pregnant women. Because animal studies are not always predictive of human responses, this drug should be used in pregnant women only if the potential benefit to the mother justifies the potential risk to the embryo or fetus.
8.3 Nursing Mothers
Olopatadine has been identified in the milk of nursing rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in the human breast milk. Nevertheless, caution should be exercised when olopatadine hydrochloride ophthalmic solution 0.2% is administered to a nursing mother.
-
11 DESCRIPTION
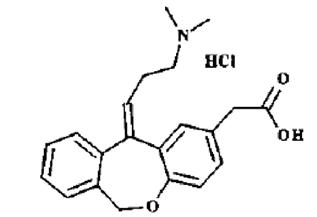
Olopatadine hydrochloride ophthalmic solution USP 0.2% is a sterile ophthalmic solution containing olopatadine for topical administration to the eyes. Olopatadine hydrochloride, USP is a white or whitish, crystalline, water-soluble powder.
The structural formula of olopatadine hydrochloride, USP is:
C21H23NO3 HCl M.W. 373.88
Chemical Name: 11-[(Z)-3-(Dimethylamino) propylidene]-6-11-dihydrodibenz[b,e] oxepin-2-acetic acid, hydrochloride.
Each mL of olopatadine hydrochloride ophthalmic solution USP for topical ocular use only, contains 2.22 mg olopatadine hydrochloride, USP equivalent to 2 mg olopatadine and has the following inactive ingredients: dibasic sodium phosphate anhydrous, edetate disodium, povidone, sodium chloride; benzalkonium chloride 0.01% (preservative); hydrochloric acid/sodium hydroxide (adjust pH); and water for injection.
It has a pH of approximately 7 and an osmolality of approximately 300 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Olopatadine is a mast cell stabilizer and a histamine H1 antagonist. Decreased chemotaxis and inhibition of eosinophil activation has also been demonstrated.
12.3 Pharmacokinetics
Systemic bioavailability data upon topical ocular administration of olopatadine hydrochloride ophthalmic solution are not available. Following topical ocular administration of olopatadine 0.15% ophthalmic solution in man, olopatadine was shown to have a low systemic exposure. Two studies in normal volunteers (totaling 24 subjects) dosed bilaterally with olopatadine 0.15% ophthalmic solution once every 12 hours for 2 weeks demonstrated plasma concentrations to be generally below the quantitation limit of the assay (< 0.5 ng/mL). Samples in which olopatadine was quantifiable were typically found within 2 hours of dosing and ranged from 0.5 to 1.3 ng/mL. The elimination half-life in plasma following oral dosing was 8 to 12 hours, and elimination was predominantly through renal excretion. Approximately 60 to 70% of the dose was recovered in the urine as parent drug. Two metabolites, the mono-desmethyl and the N-oxide, were detected at low concentrations in the urine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Olopatadine administered orally was not carcinogenic in mice and rats in doses up to 500 mg/kg/day and 200 mg/kg/day, respectively. Based on a 40 μL drop size and a 50 kg person, these doses were approximately 150,000 and 50,000 times higher than the maximum recommended ocular human dose (MROHD). No mutagenic potential was observed when olopatadine was tested in an in vitro bacterial reverse mutation (Ames) test, an in vitro mammalian chromosome aberration assay or an in vivo mouse micronucleus test. Olopatadine administered to male and female rats at oral doses of approximately 100,000 times MROHD level resulted in a slight decrease in the fertility index and reduced implantation rate; no effects on reproductive function were observed at doses of approximately 15,000 times the MROHD level.
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Olopatadine hydrochloride ophthalmic solution USP 0.2% is a clear, colorless to light yellow solution supplied in a white, low density polyethylene (LDPE) bottle with a white dropper tip, and a high density polyethylene cap.
2.5 mL fill in 5 mL bottle (NDC: 0093-7684-32)
Storage
Store at 2° to 25°C (36° to 77°F)
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
-
17 PATIENT COUNSELING INFORMATION
17.2 Sterility of Dropper Tip
Patients should be advised to not touch dropper tip to any surface, as this may contaminate the contents.
17.3 Concomitant Use of Contact Lenses
Patients should be advised not to wear a contact lens if their eyes are red. Patients should be advised that olopatadine hydrochloride ophthalmic solution should not be used to treat contact lens related irritation. Patients should also be advised to remove contact lenses prior to instillation of olopatadine hydrochloride ophthalmic solution. The preservative in olopatadine hydrochloride ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted following administration of olopatadine hydrochloride ophthalmic solution.
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Jerusalem, 9777402, Israel
Manufactured For:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Rev. A 2/2017
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0093-7684 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) EDETATE DISODIUM (UNII: 7FLD91C86K) POVIDONE K30 (UNII: U725QWY32X) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain HYDROCHLORIC ACID (UNII: QTT17582CB) May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0093-7684-32 1 in 1 CARTON 05/04/2018 1 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090848 05/04/2018 Labeler - Teva Pharmaceuticals USA, Inc. (001627975)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.