These highlights do not include all the information needed to use LEVAQUIN ® safely and effectively. See full prescribing information for LEVAQUIN ®. LEVAQUIN ® (levofloxacin) tablets, for oral use Initial U.S. Approval: 1996
Levaquin by
Drug Labeling and Warnings
Levaquin by is a Prescription medication manufactured, distributed, or labeled by REMEDYREPACK INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEVAQUIN- levofloxacin tablet, film coated
REMEDYREPACK INC.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVAQUIN
® safely and effectively. See full prescribing information for LEVAQUIN
®.
LEVAQUIN ® (levofloxacin) tablets, for oral use Initial U.S. Approval: 1996 WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVISSee full prescribing information for complete boxed warning.Fluoroquinolones, including LEVAQUIN ®, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together ( 5.1), including:
Discontinue LEVAQUIN ® immediately and avoid the use of fluoroquinolones, including LEVAQUIN ®, in patients who experience any of these serious adverse reactions ( 5.1) Fluoroquinolones, including LEVAQUIN ®, may exacerbate muscle weakness in patients with myasthenia gravis. Avoid LEVAQUIN ® in patients with a known history of myasthenia gravis [see Warnings and Precautions (5.5)] . Because fluoroquinolones, including LEVAQUIN®, have been associated with serious adverse reactions (5.1–5.14), reserve LEVAQUIN® for use in patients who have no alternative treatment options for the following indications: RECENT MAJOR CHANGES
INDICATIONS AND USAGELEVAQUIN ® is a fluoroquinolone antibacterial indicated in adults (18 years of age and older) with infections caused by designated, susceptible bacteria and in pediatric patients where indicated ( 1, 12.4).
Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of LEVAQUIN ® and other antibacterial drugs, LEVAQUIN ® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria ( 1.15). DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSTablets: 250 mg, 500 mg, and 750 mg (3) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common reactions (≥3%) were nausea, headache, diarrhea, insomnia, constipation and dizziness ( 6.2). To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-526-7736 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 4/2019 |
FULL PRESCRIBING INFORMATION
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
-
Fluoroquinolones, including LEVAQUIN
®, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together
[see
Warnings and Precautions (5.1)]
, including:
- Tendinitis and tendon rupture [see Warnings and Precautions (5.2)]
- Peripheral neuropathy [see Warnings and Precautions (5.3)]
- Central nervous system effects [see Warnings and Precautions (5.4)]
- Fluoroquinolones, including LEVAQUIN ®, may exacerbate muscle weakness in patients with myasthenia gravis. Avoid LEVAQUIN ® in patients with a known history of myasthenia gravis [see Warnings and Precautions (5.5)] .
-
Because fluoroquinolones, including LEVAQUIN®, have been associated with serious adverse reactions [see Warnings and Precautions (5.1–5.14)], reserve LEVAQUIN® for use in patients who have no alternative treatment options for the following indications:
- Uncomplicated urinary tract infection [see Indications and Usage (1.12)]
- Acute bacterial exacerbation of chronic bronchitis [see Indications and Usage (1.13)]
- Acute bacterial sinusitis [see Indications and Usage (1.14)].
1 INDICATIONS AND USAGE
1.1 Nosocomial Pneumonia
LEVAQUIN ® is indicated in adult patients for the treatment of nosocomial pneumonia due to methicillin-susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, or Streptococcus pneumoniae. Adjunctive therapy should be used as clinically indicated. Where Pseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an anti-pseudomonal β-lactam is recommended [see Clinical Studies (14.1)] .
1.2 Community-Acquired Pneumonia: 7 to 14 day Treatment Regimen
LEVAQUIN ® is indicated in adult patients for the treatment of community-acquired pneumonia due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (including multi-drug-resistant Streptococcus pneumoniae [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae [see Dosage and Administration (2.1) and Clinical Studies (14.2)] .
MDRSP isolates are isolates resistant to two or more of the following antibacterials: penicillin (MIC ≥2 mcg/mL), 2 nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
1.3 Community-Acquired Pneumonia: 5-day Treatment Regimen
LEVAQUIN ® is indicated in adult patients for the treatment of community-acquired pneumonia due to Streptococcus pneumoniae (excluding multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, or Chlamydophila pneumoniae [see Dosage and Administration (2.1) and Clinical Studies (14.3)] .
1.4 Complicated Skin and Skin Structure Infections
LEVAQUIN ® is indicated in adult patients for the treatment of complicated skin and skin structure infections due to methicillin-susceptible Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes, or Proteus mirabilis [see Clinical Studies (14.5)] .
1.5 Uncomplicated Skin and Skin Structure Infections
LEVAQUIN ® is indicated in adult patients for the treatment of uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections, due to methicillin-susceptible Staphylococcus aureus, or Streptococcus pyogenes.
1.6 Chronic Bacterial Prostatitis
LEVAQUIN ® is indicated in adult patients for the treatment of chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis, or methicillin-susceptible Staphylococcus epidermidis [see Clinical Studies (14.6)] .
1.7 Inhalational Anthrax (Post-Exposure)
LEVAQUIN ® is indicated for inhalational anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis in adults and pediatric patients, 6 months of age and older [see Dosage and Administration (2.2)] . The effectiveness of LEVAQUIN ® is based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit.
LEVAQUIN ® has not been tested in humans for the post-exposure prevention of inhalation anthrax. The safety of LEVAQUIN ® in adults for durations of therapy beyond 28 days or in pediatric patients for durations of therapy beyond 14 days has not been studied. Prolonged LEVAQUIN ® therapy should only be used when the benefit outweighs the risk [see Clinical Studies (14.9)] .
1.8 Plague
LEVAQUIN ® is indicated for treatment of plague, including pneumonic and septicemic plague, due to Yersinia pestis ( Y. pestis) and prophylaxis for plague in adults and pediatric patients, 6 months of age and older [see Dosage and Administration (2.2)] .
Efficacy studies of LEVAQUIN ® could not be conducted in humans with plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals [see Clinical Studies (14.10)] .
1.9 Complicated Urinary Tract Infections: 5-day Treatment Regimen
LEVAQUIN ® is indicated in adult patients for the treatment of complicated urinary tract infections due to Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis [see Clinical Studies (14.7)] .
1.10 Complicated Urinary Tract Infections: 10-day Treatment Regimen
LEVAQUIN ® is indicated in adult patients for the treatment of complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa [see Clinical Studies (14.8)] .
1.11 Acute Pyelonephritis: 5 or 10-day Treatment Regimen
LEVAQUIN ® is indicated in adult patients for the treatment of acute pyelonephritis caused by Escherichia coli, including cases with concurrent bacteremia [see Clinical Studies (14.7, 14.8)] .
1.12 Uncomplicated Urinary Tract Infections
LEVAQUIN ® is indicated in adult patients for the treatment of uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus.
Because fluoroquinolones, including LEVAQUIN ®, have been associated with serious adverse reactions [see Warnings and Precautions (5.1–5.14)] and for some patients uncomplicated urinary tract infection is self-limiting, reserve LEVAQUIN ® for treatment of uncomplicated urinary tract infections in patients who have no alternative treatment options.
1.13 Acute Bacterial Exacerbation of Chronic Bronchitis
LEVAQUIN ® is indicated in adult patients for the treatment of acute bacterial exacerbation of chronic bronchitis (ABECB) due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Because fluoroquinolones, including LEVAQUIN ®, have been associated with serious adverse reactions [see Warnings and Precautions (5.1–5.14)] and for some patients ABECB is self-limiting, reserve LEVAQUIN ® for treatment of ABECB in patients who have no alternative treatment options.
1.14 Acute Bacterial Sinusitis: 5-day and 10–14 day Treatment Regimens
LEVAQUIN ® is indicated in adult patients for the treatment of acute bacterial sinusitis (ABS) due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis [see Clinical Studies (14.4)] .
Because fluoroquinolones, including LEVAQUIN ®, have been associated with serious adverse reactions [see Warnings and Precautions (5.1–5.14)] and for some patients ABS is self-limiting, reserve LEVAQUIN ® for treatment of ABS in patients who have no alternative treatment options.
1.15 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of LEVAQUIN ® and other antibacterial drugs, LEVAQUIN ® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Culture and susceptibility testing
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin [see Microbiology (12.4)] . Therapy with LEVAQUIN ® may be initiated before results of these tests are known; once results become available, appropriate therapy should be selected.
As with other drugs in this class, some isolates of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with LEVAQUIN ®. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage of LEVAQUIN ® Tablets in Adult Patients with Creatinine Clearance ≥ 50 mL/minute
The usual dose of LEVAQUIN ® Tablets is 250 mg, 500 mg, or 750 mg administered orally every 24 hours, as indicated by infection and described in Table 1.
These recommendations apply to patients with creatinine clearance ≥ 50 mL/minute. For patients with creatinine clearance less than 50 mL/min, adjustments to the dosing regimen are required [see Dosage and Administration (2.3)] .
| Type of Infection * | Dosed Every 24 hours | Duration
(days) † |
|---|---|---|
|
|
||
| Nosocomial Pneumonia | 750 mg | 7 to 14 |
| Community Acquired Pneumonia ‡ | 500 mg ‡ | 7 to 14 ‡ |
| Community Acquired Pneumonia § | 750 mg § | 5 § |
| Complicated Skin and Skin Structure Infections (SSSI) | 750 mg | 7 to 14 |
| Uncomplicated SSSI | 500 mg | 7 to 10 |
| Chronic Bacterial Prostatitis | 500 mg | 28 |
| Inhalational Anthrax (Post-Exposure), adult and pediatric patients weighing 50 kg ¶,# or greater | 500 mg | 60 # |
| Pediatric patients weighing 30 kg to less than 50 kg ¶,# | see Table 2 below (2.2) | 60 # |
| Plague, adult and pediatric patients weighing 50 kg
Þ or greater
Pediatric patients weighing 30 kg to less than 50 kg | 500 mg | 10 to 14 |
| see Table 2 below (2.2) | 10 to 14 | |
| Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP) ß | 750 mg | 5 |
| Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP) à | 250 mg à | 10 à |
| Uncomplicated Urinary Tract Infection | 250 mg | 3 |
| Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB) | 500 mg | 7 |
| Acute Bacterial Sinusitis (ABS) | 750 mg | 5 |
| 500 mg | 10 to 14 | |
2.2 Dosage of LEVAQUIN ® Tablets in Pediatric Patients with Inhalational Anthrax or Plague
The dosage of LEVAQUIN ® Tablets for inhalational anthrax (post-exposure) and plague in pediatric patients who weigh 30 kg or greater is described below in Table 2. LEVAQUIN ® Tablets cannot be administered to patients who weigh less than 30 kg because of the limitations of the available strength. Alternative formulations of levofloxacin may be considered for pediatric patients who weigh less than 30 kg.
| Type of Infection * | Dose | Frequency | Duration † |
|---|---|---|---|
|
|
|||
| Inhalational Anthrax (post-exposure) ‡,§ | |||
| Pediatric patients weighing 50 kg or greater | 500 mg | every 24 hours | 60 days § |
| Pediatric patients weighing 30 kg to less than 50 kg | 250 mg | every 12 hours | 60 days § |
| Plague ¶ | |||
| Pediatric patients weighing 50 kg or greater | 500 mg | every 24 hours | 10 to 14 days |
| Pediatric patients weighing 30 kg to less than 50 kg | 250 mg | every 12 hours | 10 to 14 days |
2.3 Dosage Adjustment in Adults with Renal Impairment
Administer LEVAQUIN ® with caution in patients with renal impairment. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced in these patients.
In patients with renal impairment (creatinine clearance less than 50 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance [see Use in Specific Populations (8.6)] . No adjustment is necessary for patients with a creatinine clearance greater than or equal to 50 mL/minute.
Table 3 shows how to adjust dose based on creatinine clearance.
| Creatinine Clearance greater than or equal to 50 mL/minute | Creatinine Clearance 20 to 49 mL/minute | Creatinine Clearance 10 to 19 mL/minute | Hemodialysis or Chronic Ambulatory Peritoneal Dialysis (CAPD) |
|---|---|---|---|
| 750 mg every 24 hours | 750 mg every 48 hours | 750 mg initial dose, then 500 mg every 48 hours | 750 mg initial dose, then 500 mg every 48 hours |
| 500 mg every 24 hours | 500 mg initial dose, then 250 mg every 24 hours | 500 mg initial dose, then 250 mg every 48 hours | 500 mg initial dose, then 250 mg every 48 hours |
| 250 mg every 24 hours | No dosage adjustment required | 250 mg every 48 hours.
If treating uncomplicated UTI, then no dosage adjustment is required | No information on dosing adjustment is available |
2.4 Drug Interaction With Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
LEVAQUIN ® Tablets should be administered at least two hours before or two hours after antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine chewable/buffered tablets or the pediatric powder for oral solution [see Drug Interactions (7.1) and Patient Counseling Information (17)] .
2.5 Administration Instructions
LEVAQUIN ® Tablets can be administered without regard to food.
Hydration for Patients Receiving LEVAQUIN ® Tablets
Adequate hydration of patients receiving LEVAQUIN ® should be maintained to prevent the formation of highly concentrated urine. Crystalluria and cylindruria have been reported with quinolones [see Adverse Reactions (6.1) and Patient Counseling Information (17)] .
3 DOSAGE FORMS AND STRENGTHS
TABLETS, Film-coated, capsule-shaped
- 250 mg terra cotta pink tablets, imprinted with "250" on one side and "LEVAQUIN" on the other side
- 500 mg peach tablets, imprinted with "500" on one side and "LEVAQUIN" on the other side
- 750 mg white tablets, imprinted with "750" on one side and "LEVAQUIN" on the other side
4 CONTRAINDICATIONS
LEVAQUIN ® is contraindicated in persons with known hypersensitivity to levofloxacin, or other quinolone antibacterials [see Warnings and Precautions (5.3)] .
5 WARNINGS AND PRECAUTIONS
5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects
Fluoroquinolones, including LEVAQUIN ®, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting LEVAQUIN ®. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see Warnings and Precautions (5.2, 5.3, 5.4)] .
Discontinue LEVAQUIN ® immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including LEVAQUIN ®, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
5.2 Tendinitis and Tendon Rupture
Fluoroquinolones, including LEVAQUIN ®, have been associated with an increased risk of tendinitis and tendon rupture in all ages [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)] . This adverse reaction most frequently involves the Achilles tendon and has also been reported with the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites. Tendinitis or tendon rupture can occur within hours or days of starting LEVAQUIN ® or as long as several months after completion of fluoroquinolone therapy. Tendinitis and tendon rupture can occur bilaterally.
The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients taking fluoroquinolones who do not have the above risk factors. Discontinue LEVAQUIN ® immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug. Avoid LEVAQUIN ® in patients who have a history of tendon disorders or tendon rupture [see Adverse Reactions (6.3) and Patient Counseling Information (17)] .
5.3 Peripheral Neuropathy
Fluoroquinolones, including LEVAQUIN ®, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including LEVAQUIN ®. Symptoms may occur soon after initiation of LEVAQUIN ® and may be irreversible in some patients [see Warnings and Precautions (5.1) and Adverse Reactions (6.1, 6.2)] .
Discontinue LEVAQUIN ® immediately if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation. Avoid fluoroquinolones, including LEVAQUIN ®, in patients who have previously experienced peripheral neuropathy [see Adverse Reactions (6) and Patient Counseling Information (17)] .
5.4 Central Nervous System Effects
Psychiatric Adverse Reactions
Fluoroquinolones, including LEVAQUIN ®, have been associated with an increased risk of psychiatric adverse reactions, including: toxic psychoses, hallucinations, or paranoia; depression, or suicidal thoughts; anxiety, agitation, restlessness, or nervousness; confusion, delirium, disorientation, or disturbances in attention; insomnia or nightmares; memory impairment. Attempted or completed suicide have been reported, especially in patients with a medical history of depression, or an underlying risk factor for depression. These reactions may occur following the first dose. If these reactions occur in patients receiving LEVAQUIN ®, discontinue LEVAQUIN ® and institute appropriate measures.
Central Nervous System Adverse Reactions
Fluoroquinolones, including LEVAQUIN ®, have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (including pseudotumor cerebri), tremors, and lightheadedness. As with other fluoroquinolones, LEVAQUIN ® should be used with caution in patients with a known or suspected central nervous system (CNS) disorder that may predispose them to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose them to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction). If these reactions occur in patients receiving LEVAQUIN ®, discontinue LEVAQUIN ® and institute appropriate measures [see Adverse Reactions (6), Drug Interactions (7.4, 7.5), and Patient Counseling Information (17)] .
5.5 Exacerbation of Myasthenia Gravis
Fluoroquinolones, including LEVAQUIN ®, have neuromuscular blocking activity and may exacerbate muscle weakness in patients with myasthenia gravis. Postmarketing serious adverse reactions including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in patients with myasthenia gravis. Avoid LEVAQUIN ® in patients with a known history of myasthenia gravis [see Adverse Reactions (6.3) and Patient Counseling Information (17)].
5.6 Other Serious and Sometimes Fatal Adverse Reactions
Other serious and sometimes fatal adverse reactions, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with fluoroquinolones, including LEVAQUIN ®. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
Discontinue LEVAQUIN ® immediately at the first appearance of skin rash, jaundice, or any other sign of hypersensitivity and institute supportive measures [see Adverse Reactions (6) and Patient Counseling Information (17)] .
5.7 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with fluoroquinolones, including LEVAQUIN ®. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat, or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath, and acute respiratory distress), dyspnea, urticaria, itching, and other serious skin reactions. LEVAQUIN ® should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated [see Adverse Reactions (6) and Patient Counseling Information (17)] .
5.8 Hepatotoxicity
Post-marketing reports of severe hepatotoxicity (including acute hepatitis and fatal events) have been received for patients treated with LEVAQUIN ®. No evidence of serious drug-associated hepatotoxicity was detected in clinical trials of over 7,000 patients. Severe hepatotoxicity generally occurred within 14 days of initiation of therapy and most cases occurred within 6 days. Most cases of severe hepatotoxicity were not associated with hypersensitivity [see Warnings and Precautions (5.6)] . The majority of fatal hepatotoxicity reports occurred in patients 65 years of age or older and most were not associated with hypersensitivity. LEVAQUIN ® should be discontinued immediately if the patient develops signs and symptoms of hepatitis [see Adverse Reactions (6) and Patient Counseling Information (17)].
5.9 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including LEVAQUIN ®, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Adverse Reactions (6.2) and Patient Counseling Information (17)] .
5.10 Prolongation of the QT Interval
Some fluoroquinolones, including LEVAQUIN ®, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsade de pointes have been spontaneously reported during postmarketing surveillance in patients receiving fluoroquinolones, including LEVAQUIN ®. LEVAQUIN ® should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval [see Adverse Reactions (6.3), Use in Specific Populations (8.5), and Patient Counseling Information (17)] .
5.11 Musculoskeletal Disorders in Pediatric Patients and Arthropathic Effects in Animals
LEVAQUIN ® is indicated in pediatric patients (6 months of age and older) only for the prevention of inhalational anthrax (post-exposure) and for plague [see Indications and Usage (1.7, 1.8)] . An increased incidence of musculoskeletal disorders (arthralgia, arthritis, tendinopathy, and gait abnormality) compared to controls has been observed in pediatric patients receiving LEVAQUIN ® [see Use in Specific Populations (8.4)] .
In immature rats and dogs, the oral and intravenous administration of levofloxacin resulted in increased osteochondrosis. Histopathological examination of the weight-bearing joints of immature dogs dosed with levofloxacin revealed persistent lesions of the cartilage. Other fluoroquinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species [see Animal Toxicology and/or Pharmacology (13.2)] .
5.12 Blood Glucose Disturbances
Fluoroquinolones, including LEVAQUIN ®, have been associated with disturbances of blood glucose, including symptomatic hyperglycemia and hypoglycemia, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. Severe cases of hypoglycemia resulting in coma or death have been reported. If a hypoglycemic reaction occurs in a patient being treated with LEVAQUIN ®, discontinue LEVAQUIN ® and initiate appropriate therapy immediately [see Adverse Reactions (6.2), Drug Interactions (7.3) and Patient Counseling Information (17)] .
5.13 Photosensitivity/Phototoxicity
Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (e.g., burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, "V" area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of fluoroquinolones after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Drug therapy should be discontinued if photosensitivity/phototoxicity occurs [see Adverse Reactions (6.3) and Patient Counseling Information (17)] .
5.14 Development of Drug Resistant Bacteria
Prescribing LEVAQUIN ® in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria [see Patient Counseling Information (17)] .
6 ADVERSE REACTIONS
6.1 Serious and Otherwise Important Adverse Reactions
The following serious and otherwise important adverse drug reactions are discussed in greater detail in other sections of labeling:
- Disabling and Potentially Irreversible Serious Adverse Reactions [see Warnings and Precautions (5.1)]
- Tendinitis and Tendon Rupture [see Warnings and Precautions (5.2)]
- Peripheral Neuropathy [see Warnings and Precautions (5.3)]
- Central Nervous System Effects [see Warnings and Precautions (5.4)]
- Exacerbation of Myasthenia Gravis [see Warnings and Precautions (5.5)]
- Other Serious and Sometimes Fatal Reactions [see Warnings and Precautions (5.6)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.7)]
- Hepatotoxicity [see Warnings and Precautions (5.8)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.9)]
- Prolongation of the QT Interval [see Warnings and Precautions (5.10)]
- Musculoskeletal Disorders in Pediatric Patients [see Warnings and Precautions (5.11)]
- Blood Glucose Disturbances [see Warnings and Precautions (5.12)]
- Photosensitivity/Phototoxicity [see Warnings and Precautions (5.13)]
- Development of Drug Resistant Bacteria [see Warnings and Precautions (5.14)]
Crystalluria and cylindruria have been reported with quinolones, including LEVAQUIN ®. Therefore, adequate hydration of patients receiving LEVAQUIN ® should be maintained to prevent the formation of a highly concentrated urine [see Dosage and Administration (2.5)] .
6.2 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to LEVAQUIN ® in 7537 patients in 29 pooled Phase 3 clinical trials. The population studied had a mean age of 50 years (approximately 74% of the population was < 65 years of age), 50% were male, 71% were Caucasian, 19% were Black. Patients were treated with LEVAQUIN ® for a wide variety of infectious diseases [see Indications and Usage (1)] . Patients received LEVAQUIN ® doses of 750 mg once daily, 250 mg once daily, or 500 mg once or twice daily. Treatment duration was usually 3–14 days, and the mean number of days on therapy was 10 days.
The overall incidence, type and distribution of adverse reactions was similar in patients receiving LEVAQUIN ® doses of 750 mg once daily, 250 mg once daily, and 500 mg once or twice daily. Discontinuation of LEVAQUIN ® due to adverse drug reactions occurred in 4.3% of patients overall, 3.8% of patients treated with the 250 mg and 500 mg doses and 5.4% of patients treated with the 750 mg dose. The most common adverse drug reactions leading to discontinuation with the 250 and 500 mg doses were gastrointestinal (1.4%), primarily nausea (0.6%); vomiting (0.4%); dizziness (0.3%); and headache (0.2%). The most common adverse drug reactions leading to discontinuation with the 750 mg dose were gastrointestinal (1.2%), primarily nausea (0.6%), vomiting (0.5%); dizziness (0.3%); and headache (0.3%).
Adverse reactions occurring in ≥1% of LEVAQUIN ®-treated patients and less common adverse reactions, occurring in 0.1 to <1% of LEVAQUIN ®-treated patients, are shown in Table 4 and Table 5, respectively. The most common adverse drug reactions (≥3%) are nausea, headache, diarrhea, insomnia, constipation, and dizziness.
| System/Organ Class | Adverse Reaction | %
(N=7537) |
|---|---|---|
|
|
||
| Infections and Infestations | moniliasis | 1 |
| Psychiatric Disorders | insomnia †[see Warnings and Precautions (5.4)] | 4 |
| Nervous System Disorders | ||
| headache | 6 | |
| dizziness [see Warnings and Precautions (5.4)] | 3 | |
| Respiratory, Thoracic and Mediastinal Disorders | dyspnea [see Warnings and Precautions (5.7)] | 1 |
| Gastrointestinal Disorders | nausea | 7 |
| diarrhea | 5 | |
| constipation | 3 | |
| abdominal pain | 2 | |
| vomiting | 2 | |
| dyspepsia | 2 | |
| Skin and Subcutaneous Tissue Disorders | rash [see Warnings and Precautions (5.7)] | 2 |
| pruritus | 1 | |
| Reproductive System and Breast Disorders | Vaginitis | 1 ‡ |
| General Disorders and Administration Site Conditions | edema | 1 |
| injection site reaction | 1 | |
| chest pain | 1 | |
| System/Organ Class | Adverse Reaction |
|---|---|
|
|
|
| Infections and Infestations | genital moniliasis |
| Blood and Lymphatic System Disorders | anemia
thrombocytopenia granulocytopenia [see Warnings and Precautions (5.6)] |
| Immune System Disorders | allergic reaction [see Warnings and Precautions (5.6, 5.7)] |
| Metabolism and Nutrition Disorders | hyperglycemia
hypoglycemia [see Warnings and Precautions (5.12)] hyperkalemia |
| Psychiatric Disorders | anxiety
agitation confusion depression hallucination nightmare * [see Warnings and Precautions (5.4)] sleep disorder * anorexia abnormal dreaming * |
| Nervous System Disorders | tremor
convulsions [see Warnings and Precautions (5.4)] paresthesia [see Warnings and Precautions (5.3)] vertigo hypertonia hyperkinesias abnormal gait somnolence * syncope |
| Respiratory, Thoracic and Mediastinal Disorders | epistaxis |
| Cardiac Disorders | cardiac arrest
palpitation ventricular tachycardia ventricular arrhythmia |
| Vascular Disorders | phlebitis |
| Gastrointestinal Disorders | gastritis
stomatitis pancreatitis esophagitis gastroenteritis glossitis pseudomembranous/ C. difficile colitis [see Warnings and Precautions (5.9)] |
| Hepatobiliary Disorders | abnormal hepatic function
increased hepatic enzymes increased alkaline phosphatase |
| Skin and Subcutaneous Tissue Disorders | urticaria [see Warnings and Precautions (5.7)] |
| Musculoskeletal and Connective Tissue Disorders | arthralgia
tendinitis [see Warnings and Precautions (5.2)] myalgia skeletal pain |
| Renal and Urinary Disorders | abnormal renal function
acute renal failure [see Warnings and Precautions (5.6)] |
In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with quinolones, including LEVAQUIN ®. The relationship of the drugs to these events is not presently established.
6.3 Postmarketing Experience
Table 6 lists adverse reactions that have been identified during post-approval use of LEVAQUIN ®. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
| System/Organ Class | Adverse Reaction |
|---|---|
| Blood and Lymphatic System Disorders | pancytopenia
aplastic anemia leukopenia hemolytic anemia [see Warnings and Precautions (5.6)] eosinophilia |
| Immune System Disorders | hypersensitivity reactions, sometimes fatal including:
anaphylactic/anaphylactoid reactions anaphylactic shock angioneurotic edema serum sickness [see Warnings and Precautions (5.6, 5.7)] |
| Psychiatric Disorders | psychosis
paranoia isolated reports of suicidal ideation, suicide attempt and completed suicide [see Warnings and Precautions (5.4)] |
| Nervous System Disorders | exacerbation of myasthenia gravis
[see
Warnings and Precautions (5.5)]
anosmia ageusia parosmia dysgeusia peripheral neuropathy (may be irreversible) [see Warnings and Precautions (5.3)] isolated reports of encephalopathy abnormal electroencephalogram (EEG) dysphonia pseudotumor cerebri [see Warnings and Precautions (5.4)] |
| Eye Disorders | uveitis
vision disturbance, including diplopia visual acuity reduced vision blurred scotoma |
| Ear and Labyrinth Disorders | hypoacusis
tinnitus |
| Cardiac Disorders | isolated reports of torsade de pointes
electrocardiogram QT prolonged [see Warnings and Precautions (5.10)] tachycardia |
| Vascular Disorders | vasodilatation |
| Respiratory, Thoracic and Mediastinal Disorders | isolated reports of allergic pneumonitis [see Warnings and Precautions (5.6)] |
| Hepatobiliary Disorders | hepatic failure (including fatal cases)
hepatitis jaundice [see Warnings and Precautions (5.6), (5.8)] |
| Skin and Subcutaneous Tissue Disorders | bullous eruptions to include:
Stevens-Johnson Syndrome toxic epidermal necrolysis Acute Generalized Exanthematous Pustulosis (AGEP) fixed drug eruptions erythema multiforme [see Warnings and Precautions (5.6)] photosensitivity/phototoxicity reaction [see Warnings and Precautions (5.13)] leukocytoclastic vasculitis |
| Musculoskeletal and Connective Tissue Disorders | tendon rupture
[see
Warnings and Precautions (5.2)]
muscle injury, including rupture rhabdomyolysis |
| Renal and Urinary Disorders | interstitial nephritis [see Warnings and Precautions (5.6)] |
| General Disorders and Administration Site Conditions | multi-organ failure
pyrexia |
| Investigations | prothrombin time prolonged
international normalized ratio prolonged muscle enzymes increased |
7 DRUG INTERACTIONS
7.1 Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
While the chelation by divalent cations is less marked than with other fluoroquinolones, concurrent administration of LEVAQUIN ® Tablets with antacids containing magnesium, or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. Tablets with antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine may substantially interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least two hours before or two hours after oral LEVAQUIN ® administration.
7.2 Warfarin
No significant effect of LEVAQUIN ® on the peak plasma concentrations, AUC, and other disposition parameters for R- and S- warfarin was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of warfarin on levofloxacin absorption and disposition was observed. However, there have been reports during the postmarketing experience in patients that LEVAQUIN ® enhances the effects of warfarin. Elevations of the prothrombin time in the setting of concurrent warfarin and LEVAQUIN ® use have been associated with episodes of bleeding. Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored if LEVAQUIN ® is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding [see Adverse Reactions (6.3) and Patient Counseling Information (17)] .
7.3 Antidiabetic Agents
Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with fluoroquinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered [see Warnings and Precautions (5.12), Adverse Reactions (6.2), and Patient Counseling Information (17)] .
7.4 Non-Steroidal Anti-Inflammatory Drugs
The concomitant administration of a non-steroidal anti-inflammatory drug with a fluoroquinolone, including LEVAQUIN ®, may increase the risk of CNS stimulation and convulsive seizures [see Warnings and Precautions (5.4)] .
7.5 Theophylline
No significant effect of LEVAQUIN ® on the plasma concentrations, AUC, and other disposition parameters for theophylline was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of theophylline on levofloxacin absorption and disposition was observed. However, concomitant administration of other fluoroquinolones with theophylline has resulted in prolonged elimination half-life, elevated serum theophylline levels, and a subsequent increase in the risk of theophylline-related adverse reactions in the patient population. Therefore, theophylline levels should be closely monitored and appropriate dosage adjustments made when LEVAQUIN ® is co-administered. Adverse reactions, including seizures, may occur with or without an elevation in serum theophylline levels [see Warnings and Precautions (5.4)] .
7.6 Cyclosporine
No significant effect of LEVAQUIN ® on the peak plasma concentrations, AUC, and other disposition parameters for cyclosporine was detected in a clinical study involving healthy volunteers. However, elevated serum levels of cyclosporine have been reported in the patient population when co-administered with some other fluoroquinolones. Levofloxacin C max and k e were slightly lower while T max and t ½ were slightly longer in the presence of cyclosporine than those observed in other studies without concomitant medication. The differences, however, are not considered to be clinically significant. Therefore, no dosage adjustment is required for LEVAQUIN ® or cyclosporine when administered concomitantly.
7.7 Digoxin
No significant effect of LEVAQUIN ® on the peak plasma concentrations, AUC, and other disposition parameters for digoxin was detected in a clinical study involving healthy volunteers. Levofloxacin absorption and disposition kinetics were similar in the presence or absence of digoxin. Therefore, no dosage adjustment for LEVAQUIN ® or digoxin is required when administered concomitantly.
7.8 Probenecid and Cimetidine
No significant effect of probenecid or cimetidine on the C max of levofloxacin was observed in a clinical study involving healthy volunteers. The AUC and t ½ of levofloxacin were higher while CL/F and CL R were lower during concomitant treatment of LEVAQUIN ® with probenecid or cimetidine compared to LEVAQUIN ® alone. However, these changes do not warrant dosage adjustment for LEVAQUIN ® when probenecid or cimetidine is co-administered.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Levofloxacin was not teratogenic in rats at doses as high as 810 mg/kg/day which corresponds to 9.4 times the highest recommended oral human dose based upon relative body surface area. The oral dose of 810 mg/kg/day to rats caused decreased fetal body weight and increased fetal mortality. No teratogenicity was observed when rabbits were dosed orally as high as 50 mg/kg/day which corresponds to 1.1 times the highest recommended oral human dose based upon relative body surface area.
There are, however, no adequate and well-controlled studies in pregnant women. LEVAQUIN ® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Based on data on other fluoroquinolones and very limited data on LEVAQUIN ®, it can be presumed that levofloxacin will be excreted in human milk. Because of the potential for serious adverse reactions from LEVAQUIN ® in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species. [see Warnings and Precautions (5.11) and Animal Toxicology and/or Pharmacology (13.2)] .
Inhalational Anthrax (Post-Exposure)
Levofloxacin is indicated in pediatric patients 6 months of age and older, for inhalational anthrax (post-exposure). The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate. The safety of levofloxacin in pediatric patients treated for more than 14 days has not been studied [see Indications and Usage (1.7), Dosage and Administration (2.2) and Clinical Studies (14.9)] .
Plague
Levofloxacin is indicated in pediatric patients, 6 months of age and older, for treatment of plague, including pneumonic and septicemic plague due to Yersinia pestis ( Y. pestis) and prophylaxis for plague. Efficacy studies of LEVAQUIN ® could not be conducted in humans with pneumonic plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals. The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate [see Indications and Usage (1.8), Dosage and Administration (2.2) and Clinical Studies (14.10)].
Safety and effectiveness of LEVAQUIN ® in pediatric patients below the age of six months have not been established.
Pharmacokinetics following intravenous administration
The pharmacokinetics of levofloxacin following a single intravenous dose were investigated in pediatric patients ranging in age from six months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients resulting in lower plasma exposures than adults for a given mg/kg dose [see Clinical Pharmacology (12.3) and Clinical Studies (14.9)] .
Dosage in Pediatric Patients with Inhalational Anthrax or Plague
For the recommended LEVAQUIN ® tablet dosage in pediatric patients with inhalational anthrax or plague, see Dosage and Administration (2.2). LEVAQUIN ® Tablets cannot be administered to pediatric patients who weigh less than 30 kg because of the limitations of the available strengths. Alternative formulations of levofloxacin may be considered for pediatric patients who weigh less than 30 kg.
Adverse Reactions
In clinical trials, 1534 pediatric patients (6 months to 16 years of age) were treated with oral and intravenous LEVAQUIN ®. Pediatric patients 6 months to 5 years of age received LEVAQUIN ® 10 mg/kg twice a day and pediatric patients greater than 5 years of age received 10 mg/kg once a day (maximum 500 mg per day) for approximately 10 days. LEVAQUIN ® Tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)] .
A subset of pediatric patients in the clinical trials (1340 LEVAQUIN ®-treated and 893 non-fluoroquinolone-treated) enrolled in a prospective, long-term surveillance study to assess the incidence of protocol-defined musculoskeletal disorders (arthralgia, arthritis, tendinopathy, gait abnormality) during 60 days and 1 year following the first dose of the study drug. Pediatric patients treated with LEVAQUIN ® had a significantly higher incidence of musculoskeletal disorders when compared to the non-fluoroquinolone-treated children as illustrated in Table 7. LEVAQUIN ® Tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)].
| Follow-up Period | LEVAQUIN
®
N = 1340 | Non-Fluoroquinolone
*
N = 893 | p-value † |
|---|---|---|---|
|
|
|||
| 60 days | 28 (2.1%) | 8 (0.9%) | p = 0.038 |
| 1 year ‡ | 46 (3.4%) | 16 (1.8%) | p = 0.025 |
Arthralgia was the most frequently occurring musculoskeletal disorder in both treatment groups. Most of the musculoskeletal disorders in both groups involved multiple weight-bearing joints. Disorders were moderate in 8/46 (17%) children and mild in 35/46 (76%) LEVAQUIN ®-treated pediatric patients and most were treated with analgesics. The median time to resolution was 7 days for LEVAQUIN ®-treated pediatric patients and 9 for non-fluoroquinolone-treated children (approximately 80% resolved within 2 months in both groups). No pediatric patient had a severe or serious disorder and all musculoskeletal disorders resolved without sequelae.
Vomiting and diarrhea were the most frequently reported adverse reactions, occurring in similar frequency in the LEVAQUIN ®-treated and non-fluoroquinolone-treated pediatric patients.
In addition to the adverse reactions reported in pediatric patients in clinical trials, adverse reactions reported in adults during clinical trials or post-marketing experience [see Adverse Reactions (6)] may also be expected to occur in pediatric patients.
8.5 Geriatric Use
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as LEVAQUIN ®. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing LEVAQUIN ® to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue LEVAQUIN ® and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur [see Boxed Warning; Warnings and Precautions (5.2); and Adverse Reactions (6.3)] .
In Phase 3 clinical trials, 1,945 LEVAQUIN ®-treated patients (26%) were ≥ 65 years of age. Of these, 1,081 patients (14%) were between the ages of 65 and 74 and 864 patients (12%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Severe, and sometimes fatal, cases of hepatotoxicity have been reported post-marketing in association with LEVAQUIN ®. The majority of fatal hepatotoxicity reports occurred in patients 65 years of age or older and most were not associated with hypersensitivity. LEVAQUIN ® should be discontinued immediately if the patient develops signs and symptoms of hepatitis [see Warnings and Precautions (5.8)] .
Elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, precaution should be taken when using LEVAQUIN ® with concomitant drugs that can result in prolongation of the QT interval (e.g., Class IA or Class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g., known QT prolongation, uncorrected hypokalemia) [see Warnings and Precautions (5.10)] .
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However, since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)] .
8.6 Renal Impairment
Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in patients with renal impairment (creatinine clearance < 50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of LEVAQUIN ® are not required following hemodialysis or CAPD [see Dosage and Administration (2.3)] .
10 OVERDOSAGE
In the event of an acute overdosage, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
LEVAQUIN ® exhibits a low potential for acute toxicity. Mice, rats, dogs and monkeys exhibited the following clinical signs after receiving a single high dose of LEVAQUIN ®: ataxia, ptosis, decreased locomotor activity, dyspnea, prostration, tremors, and convulsions. Doses in excess of 1500 mg/kg orally and 250 mg/kg IV produced significant mortality in rodents.
11 DESCRIPTION
LEVAQUIN ® Tablets are synthetic antibacterial agents for oral administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The chemical name is (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate.
Figure 1: The Chemical Structure of Levofloxacin
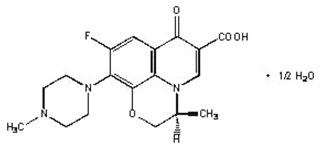
The empirical formula is C 18H 20FN 3O 4 ∙ ½ H 2O and the molecular weight is 370.38. Levofloxacin is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine.
The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin is essentially constant (approximately 100 mg/mL). Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9.
Levofloxacin has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Al +3>Cu +2>Zn +2>Mg +2>Ca +2.
LEVAQUIN ® Tablets are available as film-coated tablets and contain the following inactive ingredients:
- 250 mg (as expressed in the anhydrous form): crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, synthetic red iron oxide and titanium dioxide.
- 500 mg (as expressed in the anhydrous form): crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, synthetic red and yellow iron oxides and titanium dioxide.
- 750 mg (as expressed in the anhydrous form): crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Levofloxacin is a member of the fluoroquinolone class of antibacterial agents [see Microbiology (12.4)] .
12.3 Pharmacokinetics
The mean ± SD pharmacokinetic parameters of levofloxacin determined under single and steady-state conditions following administration of the oral tablets, are summarized in Table 8.
| Regimen | C
max
(mcg/mL) | T
max
(h) | AUC
(mcg∙h/mL) | CL/F
*
(mL/min) | Vd/F
†
(L) | t
1/2
(h) | CL
R
(mL/min) |
|---|---|---|---|---|---|---|---|
| ND=not determined. | |||||||
|
|
|||||||
| Single dose | |||||||
| 250 mg oral tablet ‡ | 2.8 ± 0.4 | 1.6 ± 1.0 | 27.2 ± 3.9 | 156 ± 20 | ND | 7.3 ± 0.9 | 142 ± 21 |
| 500 mg oral tablet ‡§ | 5.1 ± 0.8 | 1.3 ± 0.6 | 47.9 ± 6.8 | 178 ± 28 | ND | 6.3 ± 0.6 | 103 ± 30 |
| 750 mg oral tablet ¶§ | 9.3 ± 1.6 | 1.6 ± 0.8 | 101 ± 20 | 129 ± 24 | 83 ± 17 | 7.5 ± 0.9 | ND |
| Multiple dose | |||||||
| 500 mg every 24h oral tablet ‡ | 5.7 ± 1.4 | 1.1 ± 0.4 | 47.5 ± 6.7 | 175 ± 25 | 102 ± 22 | 7.6 ± 1.6 | 116 ± 31 |
| 750 mg every 24h oral tablet ¶ | 8.6 ± 1.9 | 1.4 ± 0.5 | 90.7 ± 17.6 | 143 ± 29 | 100 ± 16 | 8.8 ± 1.5 | 116 ± 28 |
| 500 mg oral tablet single dose, effects of gender and age: | |||||||
| Male # | 5.5 ± 1.1 | 1.2 ± 0.4 | 54.4 ± 18.9 | 166 ± 44 | 89 ± 13 | 7.5 ± 2.1 | 126 ± 38 |
| Female Þ | 7.0 ± 1.6 | 1.7 ± 0.5 | 67.7 ± 24.2 | 136 ± 44 | 62 ± 16 | 6.1 ± 0.8 | 106 ± 40 |
| Young ß | 5.5 ± 1.0 | 1.5 ± 0.6 | 47.5 ± 9.8 | 182 ± 35 | 83 ± 18 | 6.0 ± 0.9 | 140 ± 33 |
| Elderly à | 7.0 ± 1.6 | 1.4 ± 0.5 | 74.7 ± 23.3 | 121 ± 33 | 67 ± 19 | 7.6 ± 2.0 | 91 ± 29 |
| 500 mg oral single dose tablet, patients with renal impairment: | |||||||
| CLCR 50–80 mL/min | 7.5 ± 1.8 | 1.5 ± 0.5 | 95.6 ± 11.8 | 88 ± 10 | ND | 9.1 ± 0.9 | 57 ± 8 |
| CLCR 20–49 mL/min | 7.1 ± 3.1 | 2.1 ± 1.3 | 182.1 ± 62.6 | 51 ± 19 | ND | 27 ± 10 | 26 ± 13 |
| CLCR <20 mL/min | 8.2 ± 2.6 | 1.1 ± 1.0 | 263.5 ± 72.5 | 33 ± 8 | ND | 35 ± 5 | 13 ± 3 |
| Hemodialysis | 5.7 ± 1.0 | 2.8 ± 2.2 | ND | ND | ND | 76 ± 42 | ND |
| CAPD | 6.9 ± 2.3 | 1.4 ± 1.1 | ND | ND | ND | 51 ± 24 | ND |
Levofloxacin pharmacokinetics are linear and predictable after single and multiple oral or IV dosing regimens. Steady-state conditions are reached within 48 hours following a 500 mg or 750 mg once-daily dosage regimen. The mean ± SD peak and trough plasma concentrations attained following multiple once-daily oral dosage regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 mcg/mL after the 500 mg doses, and 8.6 ± 1.9 and 1.1 ± 0.4 mcg/mL after the 750 mg doses, respectively. The mean ± SD peak and trough plasma concentrations attained following multiple once-daily IV regimens were approximately 6.4 ± 0.8 and 0.6 ± 0.2 mcg/mL after the 500 mg doses, and 12.1 ± 4.1 and 1.3 ± 0.71 mcg/mL after the 750 mg doses, respectively.
Absorption
Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained one to two hours after oral dosing. The absolute bioavailability of levofloxacin from a 500 mg tablet and a 750 mg tablet of LEVAQUIN ® are both approximately 99%, demonstrating complete oral absorption of levofloxacin. Following a single intravenous dose of LEVAQUIN ® to healthy volunteers, the mean ± SD peak plasma concentration attained was 6.2 ± 1.0 mcg/mL after a 500 mg dose infused over 60 minutes and 11.5 ± 4.0 mcg/mL after a 750 mg dose infused over 90 minutes. Oral administration of a 500 mg dose of LEVAQUIN ® with food prolongs the time to peak concentration by approximately 1 hour and decreases the peak concentration by approximately 14% following tablet and approximately 25% following oral solution administration. Therefore, LEVAQUIN ® Tablets can be administered without regard to food.
The plasma concentration profile of levofloxacin after IV administration is similar and comparable in extent of exposure (AUC) to that observed for LEVAQUIN ® Tablets when equal doses (mg/mg) are administered. Therefore, the oral and IV routes of administration can be considered interchangeable .
Distribution
The mean volume of distribution of levofloxacin generally ranges from 74 to 112 L after single and multiple 500 mg or 750 mg doses, indicating widespread distribution into body tissues. Levofloxacin reaches its peak levels in skin tissues and in blister fluid of healthy subjects at approximately 3 hours after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple once-daily oral administration of 750 mg and 500 mg doses of LEVAQUIN ®, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2- to 5-fold higher than plasma concentrations and ranged from approximately 2.4 to 11.3 mcg/g over a 24-hour period after a single 500 mg oral dose.
In vitro, over a clinically relevant range (1 to 10 mcg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24 to 38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Elimination
Metabolism
Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered as unchanged drug in urine within 48 hours, whereas less than 4% of the dose was recovered in feces in 72 hours. Less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Excretion
Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6 to 8 hours following single or multiple doses of levofloxacin given orally or intravenously. The mean apparent total body clearance and renal clearance range from approximately 144 to 226 mL/min and 96 to 142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid results in approximately 24% and 35% reduction in the levofloxacin renal clearance, respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving LEVAQUIN ®.
Specific Populations
Geriatric Patients
There are no significant differences in levofloxacin pharmacokinetics between young and elderly subjects when the subjects' differences in creatinine clearance are taken into consideration. Following a 500 mg oral dose of LEVAQUIN ® to healthy elderly subjects (66–80 years of age), the mean terminal plasma elimination half-life of levofloxacin was about 7.6 hours, as compared to approximately 6 hours in younger adults. The difference was attributable to the variation in renal function status of the subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by age. LEVAQUIN ® dose adjustment based on age alone is not necessary [see Use in Specific Populations (8.5)] .
Pediatric Patients
The pharmacokinetics of levofloxacin following a single 7 mg/kg intravenous dose were investigated in pediatric patients ranging in age from 6 months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients, resulting in lower plasma exposures than adults for a given mg/kg dose. Subsequent pharmacokinetic analyses predicted that a dosage regimen of 8 mg/kg every 12 hours (not to exceed 250 mg per dose) for pediatric patients 6 months to 17 years of age would achieve comparable steady state plasma exposures (AUC 0–24 and C max) to those observed in adult patients administered 500 mg of levofloxacin once every 24 hours. LEVAQUIN ® Tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)] .
Male and Female Subjects
There are no significant differences in levofloxacin pharmacokinetics between male and female subjects when subjects' differences in creatinine clearance are taken into consideration. Following a 500 mg oral dose of LEVAQUIN ® to healthy male subjects, the mean terminal plasma elimination half-life of levofloxacin was about 7.5 hours, as compared to approximately 6.1 hours in female subjects. This difference was attributable to the variation in renal function status of the male and female subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by the gender of the subjects. Dose adjustment based on gender alone is not necessary.
Racial or Ethnic Groups
The effect of race on levofloxacin pharmacokinetics was examined through a covariate analysis performed on data from 72 subjects: 48 white and 24 non-white. The apparent total body clearance and apparent volume of distribution were not affected by the race of the subjects.
Patients with Renal Impairment
Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in adult patients with impaired renal function (creatinine clearance < 50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of LEVAQUIN ® are not required following hemodialysis or CAPD [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)] .
Patients with Hepatic Impairment
Pharmacokinetic studies in hepatically impaired patients have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment [see Use in Specific Populations (8.7)] .
Drug Interaction Studies
The potential for pharmacokinetic drug interactions between LEVAQUIN ® and antacids, warfarin, theophylline, cyclosporine, digoxin, probenecid, and cimetidine has been evaluated [see Drug Interactions (7)] .
12.4 Microbiology
Mechanism of Action
Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair and recombination.
Resistance
Fluoroquinolone resistance can arise through mutations in defined regions of DNA gyrase or topoisomerase IV, termed the Quinolone-Resistance Determining Regions (QRDRs), or through altered efflux.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from aminoglycosides, macrolides and β-lactam antibiotics, including penicillins. Fluoroquinolones may, therefore, be active against bacteria resistant to these antimicrobials.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10 -9 to 10 -10). Cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Antimicrobial Activity
Levofloxacin has in vitro activity against Gram-negative and Gram-positive bacteria.
Levofloxacin has been shown to be active against most isolates of the following bacteria both in vitro and in clinical infections as described in Indications and Usage (1):
Aerobic bacteria
Gram-Positive Bacteria
Enterococcus faecalis
Staphylococcus aureus (methicillin-susceptible isolates)
Staphylococcus epidermidis (methicillin-susceptible isolates)
Staphylococcus saprophyticus
Streptococcus pneumoniae (including multi-drug resistant isolates [MDRSP] 1)
Streptococcus pyogenes
Gram-Negative Bacteria
Enterobacter cloacae
Escherichia coli
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella pneumoniae
Legionella pneumophila
Moraxella catarrhalis
Proteus mirabilis
Pseudomonas aeruginosa
Serratia marcescens
Other microorganisms
Chlamydophila pneumoniae
Mycoplasma pneumoniae
The following in vitro data are available, but their clinical significance is unknown: Levofloxacin exhibits in vitro minimum inhibitory concentrations (MIC values) of 2 mcg/mL or less against most (≥90%) isolates of the following microorganisms; however, the safety and effectiveness of LEVAQUIN ® in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Aerobic bacteria
Gram-Positive Bacteria
Staphylococcus haemolyticus
β-hemolytic Streptococcus (Group C/F)
β-hemolytic Streptococcus (Group G)
Streptococcus agalactiae
Streptococcus milleri
Viridans group streptococci
Bacillus anthracis
Gram-Negative Bacteria
Acinetobacter baumannii
Acinetobacter lwoffii
Bordetella pertussis
Citrobacter koseri
Citrobacter freundii
Enterobacter aerogenes
Enterobacter sakazakii
Klebsiella oxytoca
Morganella morganii
Pantoea agglomerans
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Pseudomonas fluorescens
Yersinia pestis
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a lifetime bioassay in rats, levofloxacin exhibited no carcinogenic potential following daily dietary administration for 2 years; the highest dose (100 mg/kg/day ) was 1.4 times the highest recommended human dose (750 mg) based upon relative body surface area. Levofloxacin did not shorten the time to tumor development of UV-induced skin tumors in hairless albino (Skh-1) mice at any levofloxacin dose level and was therefore not photo-carcinogenic under conditions of this study. Dermal levofloxacin concentrations in the hairless mice ranged from 25 to 42 mcg/g at the highest levofloxacin dose level (300 mg/kg/day) used in the photo-carcinogenicity study. By comparison, dermal levofloxacin concentrations in human subjects receiving 750 mg of LEVAQUIN ® averaged approximately 11.8 mcg/g at C max.
Levofloxacin was not mutagenic in the following assays: Ames bacterial mutation assay ( S. typhimurium and E. coli), CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and sister chromatid exchange (CHL/IU cell line) assays.
Levofloxacin caused no impairment of fertility or reproductive performance in rats at oral doses as high as 360 mg/kg/day, corresponding to 4.2 times the highest recommended human dose based upon relative body surface area and intravenous doses as high as 100 mg/kg/day, corresponding to 1.2 times the highest recommended human dose based upon relative body surface area.
13.2 Animal Toxicology and/or Pharmacology
Levofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested [see Warnings and Precautions (5.11)] . In immature dogs (4–5 months old), oral doses of 10 mg/kg/day for 7 days and intravenous doses of 4 mg/kg/day for 14 days of levofloxacin resulted in arthropathic lesions. Administration at oral doses of 300 mg/kg/day for 7 days and intravenous doses of 60 mg/kg/day for 4 weeks produced arthropathy in juvenile rats. Three-month old beagle dogs dosed orally with levofloxacin at 40 mg/kg/day exhibited clinically severe arthrotoxicity resulting in the termination of dosing at Day 8 of a 14-day dosing routine. Slight musculoskeletal clinical effects, in the absence of gross pathological or histopathological effects, resulted from the lowest dose level of 2.5 mg/kg/day (approximately 0.2-fold the pediatric dose based upon AUC comparisons). Synovitis and articular cartilage lesions were observed at the 10 and 40 mg/kg dose levels (approximately 0.7-fold and 2.4-fold the pediatric dose, respectively, based on AUC comparisons). Articular cartilage gross pathology and histopathology persisted to the end of the 18-week recovery period for those dogs from the 10 and 40 mg/kg/day dose levels.
When tested in a mouse ear swelling bioassay, levofloxacin exhibited phototoxicity similar in magnitude to ofloxacin, but less phototoxicity than other quinolones.
While crystalluria has been observed in some intravenous rat studies, urinary crystals are not formed in the bladder, being present only after micturition and are not associated with nephrotoxicity.
In mice, the CNS stimulatory effect of quinolones is enhanced by concomitant administration of non-steroidal anti-inflammatory drugs.
In dogs, levofloxacin administered at 6 mg/kg or higher by rapid intravenous injection produced hypotensive effects. These effects were considered to be related to histamine release.
In vitro and in vivo studies in animals indicate that levofloxacin is neither an enzyme inducer nor inhibitor in the human therapeutic plasma concentration range; therefore, no drug metabolizing enzyme-related interactions with other drugs or agents are anticipated.
14 CLINICAL STUDIES
14.1 Nosocomial Pneumonia
Adult patients with clinically and radiologically documented nosocomial pneumonia were enrolled in a multicenter, randomized, open-label study comparing intravenous LEVAQUIN ® (750 mg once daily) followed by oral LEVAQUIN ® (750 mg once daily) for a total of 7–15 days to intravenous imipenem/cilastatin (500–1000 mg every 6–8 hours daily) followed by oral ciprofloxacin (750 mg every 12 hours daily) for a total of 7–15 days. LEVAQUIN ®-treated patients received an average of 7 days of intravenous therapy (range: 1–16 days); comparator-treated patients received an average of 8 days of intravenous therapy (range: 1–19 days).
Overall, in the clinically and microbiologically evaluable population, adjunctive therapy was empirically initiated at study entry in 56 of 93 (60.2%) patients in the LEVAQUIN ® arm and 53 of 94 (56.4%) patients in the comparator arm. The average duration of adjunctive therapy was 7 days in the LEVAQUIN ® arm and 7 days in the comparator. In clinically and microbiologically evaluable patients with documented Pseudomonas aeruginosa infection, 15 of 17 (88.2%) received ceftazidime (N = 11) or piperacillin/tazobactam (N = 4) in the LEVAQUIN ® arm and 16 of 17 (94.1%) received an aminoglycoside in the comparator arm. Overall, in clinically and microbiologically evaluable patients, vancomycin was added to the treatment regimen of 37 of 93 (39.8%) patients in the LEVAQUIN ® arm and 28 of 94 (29.8%) patients in the comparator arm for suspected methicillin-resistant S. aureus infection.
Clinical success rates in clinically and microbiologically evaluable patients at the post-therapy visit (primary study endpoint assessed on day 3–15 after completing therapy) were 58.1% for LEVAQUIN ® and 60.6% for comparator. The 95% CI for the difference of response rates (LEVAQUIN ® minus comparator) was [-17.2, 12.0]. The microbiological eradication rates at the posttherapy visit were 66.7% for LEVAQUIN ® and 60.6% for comparator. The 95% CI for the difference of eradication rates (LEVAQUIN ® minus comparator) was [-8.3, 20.3]. Clinical success and microbiological eradication rates by pathogen are detailed in Table 9.
| Pathogen | N | LEVAQUIN ® No. (%) of Patients Microbiologic/Clinical Outcomes | N | Imipenem/Cilastatin No. (%) of Patients Microbiologic/Clinical Outcomes |
|---|---|---|---|---|
|
|
||||
| MSSA * | 21 | 14 (66.7)/13 (61.9) | 19 | 13 (68.4)/15 (78.9) |
| P. aeruginosa † | 17 | 10 (58.8)/11 (64.7) | 17 | 5 (29.4)/7 (41.2) |
| S. marcescens | 11 | 9 (81.8)/7 (63.6) | 7 | 2 (28.6)/3 (42.9) |
| E. coli | 12 | 10 (83.3)/7 (58.3) | 11 | 7 (63.6)/8 (72.7) |
| K. pneumoniae ‡ | 11 | 9 (81.8)/5 (45.5) | 7 | 6 (85.7)/3 (42.9) |
| H. influenzae | 16 | 13 (81.3)/10 (62.5) | 15 | 14 (93.3)/11 (73.3) |
| S. pneumoniae | 4 | 3 (75.0)/3 (75.0) | 7 | 5 (71.4)/4 (57.1) |
14.2 Community-Acquired Pneumonia: 7–14 day Treatment Regimen
Adult inpatients and outpatients with a diagnosis of community-acquired bacterial pneumonia were evaluated in 2 pivotal clinical studies. In the first study, 590 patients were enrolled in a prospective, multi-center, unblinded randomized trial comparing LEVAQUIN ® 500 mg once daily orally or intravenously for 7 to 14 days to ceftriaxone 1 to 2 grams intravenously once or in equally divided doses twice daily followed by cefuroxime axetil 500 mg orally twice daily for a total of 7 to 14 days. Patients assigned to treatment with the control regimen were allowed to receive erythromycin (or doxycycline if intolerant of erythromycin) if an infection due to atypical pathogens was suspected or proven. Clinical and microbiologic evaluations were performed during treatment, 5 to 7 days posttherapy, and 3 to 4 weeks posttherapy. Clinical success (cure plus improvement) with LEVAQUIN ® at 5 to 7 days posttherapy, the primary efficacy variable in this study, was superior (95%) to the control group (83%). The 95% CI for the difference of response rates (LEVAQUIN ® minus comparator) was [-6, 19]. In the second study, 264 patients were enrolled in a prospective, multi-center, non-comparative trial of 500 mg LEVAQUIN ® administered orally or intravenously once daily for 7 to 14 days. Clinical success for clinically evaluable patients was 93%. For both studies, the clinical success rate in patients with atypical pneumonia due to Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila were 96%, 96%, and 70%, respectively. Microbiologic eradication rates across both studies are presented in Table 10.
| Pathogen | No. Pathogens | Bacteriological Eradication Rate (%) |
|---|---|---|
| H. influenzae | 55 | 98 |
| S. pneumoniae | 83 | 95 |
| S. aureus | 17 | 88 |
| M. catarrhalis | 18 | 94 |
| H. parainfluenzae | 19 | 95 |
| K. pneumoniae | 10 | 100.0 |
Community-Acquired Pneumonia Due to Multi-Drug Resistant Streptococcus pneumoniae
LEVAQUIN ® was effective for the treatment of community-acquired pneumonia caused by multi-drug resistant Streptococcus pneumoniae (MDRSP). MDRSP isolates are isolates resistant to two or more of the following antibacterials: penicillin (MIC ≥2 mcg/mL), 2 nd generation cephalosporins (e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole). Of 40 microbiologically evaluable patients with MDRSP isolates, 38 patients (95.0%) achieved clinical and bacteriologic success at post-therapy. The clinical and bacterial success rates are shown in Table 11.
| Screening Susceptibility | Clinical Success | Bacteriological Success * | ||
|---|---|---|---|---|
| n/N † | % | n/N ‡ | % | |
|
|
||||
| Penicillin-resistant | 16/17 | 94.1 | 16/17 | 94.1 |
| 2nd generation Cephalosporin resistant | 31/32 | 96.9 | 31/32 | 96.9 |
| Macrolide-resistant | 28/29 | 96.6 | 28/29 | 96.6 |
| Trimethoprim/ Sulfamethoxazole resistant | 17/19 | 89.5 | 17/19 | 89.5 |
| Tetracycline-resistant | 12/12 | 100 | 12/12 | 100 |
Not all isolates were resistant to all antimicrobial classes tested. Success and eradication rates are summarized in Table 12.
| Type of Resistance | Clinical Success | Bacteriologic Eradication |
|---|---|---|
| Resistant to 2 antibacterials | 17/18 (94.4%) | 17/18 (94.4%) |
| Resistant to 3 antibacterials | 14/15 (93.3%) | 14/15 (93.3%) |
| Resistant to 4 antibacterials | 7/7 (100%) | 7/7 (100%) |
| Resistant to 5 antibacterials | 0 | 0 |
| Bacteremia with MDRSP | 8/9 (89%) | 8/9 (89%) |
14.3 Community-Acquired Pneumonia: 5-day Treatment Regimen
To evaluate the safety and efficacy of the higher dose and shorter course of LEVAQUIN ®, 528 outpatient and hospitalized adults with clinically and radiologically determined mild to severe community-acquired pneumonia were evaluated in a double-blind, randomized, prospective, multicenter study comparing LEVAQUIN ® 750 mg, IV or orally, every day for five days or LEVAQUIN ® 500 mg IV or orally, every day for 10 days.
Clinical success rates (cure plus improvement) in the clinically evaluable population were 90.9% in the LEVAQUIN ® 750 mg group and 91.1% in the LEVAQUIN ® 500 mg group. The 95% CI for the difference of response rates (LEVAQUIN ® 750 minus LEVAQUIN ® 500) was [-5.9, 5.4]. In the clinically evaluable population (31–38 days after enrollment) pneumonia was observed in 7 out of 151 patients in the LEVAQUIN ® 750 mg group and 2 out of 147 patients in the LEVAQUIN ® 500 mg group. Given the small numbers observed, the significance of this finding cannot be determined statistically. The microbiological efficacy of the 5-day regimen was documented for infections listed in Table 13.
| S. pneumoniae | 19/20 (95%) |
| Haemophilus influenzae | 12/12 (100%) |
| Haemophilus parainfluenzae | 10/10 (100%) |
| Mycoplasma pneumoniae | 26/27 (96%) |
| Chlamydophila pneumoniae | 13/15 (87%) |
14.4 Acute Bacterial Sinusitis: 5-day and 10–14 day Treatment Regimens
LEVAQUIN ® is approved for the treatment of acute bacterial sinusitis (ABS) using either 750 mg by mouth × 5 days or 500 mg by mouth once daily × 10–14 days. To evaluate the safety and efficacy of a high dose short course of LEVAQUIN ®, 780 outpatient adults with clinically and radiologically determined acute bacterial sinusitis were evaluated in a double-blind, randomized, prospective, multicenter study comparing LEVAQUIN ® 750 mg by mouth once daily for five days to LEVAQUIN ® 500 mg by mouth once daily for 10 days.
Clinical success rates (defined as complete or partial resolution of the pre-treatment signs and symptoms of ABS to such an extent that no further antibiotic treatment was deemed necessary) in the microbiologically evaluable population were 91.4% (139/152) in the LEVAQUIN ® 750 mg group and 88.6% (132/149) in the LEVAQUIN ® 500 mg group at the test-of-cure (TOC) visit (95% CI [-4.2, 10.0] for LEVAQUIN ® 750 mg minus LEVAQUIN ® 500 mg).
Rates of clinical success by pathogen in the microbiologically evaluable population who had specimens obtained by antral tap at study entry showed comparable results for the five- and ten-day regimens at the test-of-cure visit 22 days post treatment (see Table 14).
| Pathogen | LEVAQUIN ® 750 mg × 5 days | LEVAQUIN ® 500 mg × 10 days |
|---|---|---|
|
|
||
| Streptococcus pneumoniae * | 25/27 (92.6%) | 26/27 (96.3%) |
| Haemophilus influenzae * | 19/21 (90.5%) | 25/27 (92.6%) |
| Moraxella catarrhalis * | 10/11 (90.9%) | 13/13 (100%) |
14.5 Complicated Skin and Skin Structure Infections
Three hundred ninety-nine patients were enrolled in an open-label, randomized, comparative study for complicated skin and skin structure infections. The patients were randomized to receive either LEVAQUIN ® 750 mg once daily (IV followed by oral), or an approved comparator for a median of 10 ± 4.7 days. As is expected in complicated skin and skin structure infections, surgical procedures were performed in the LEVAQUIN ® and comparator groups. Surgery (incision and drainage or debridement) was performed on 45% of the LEVAQUIN ®-treated patients and 44% of the comparator-treated patients, either shortly before or during antibiotic treatment and formed an integral part of therapy for this indication.
Among those who could be evaluated clinically 2–5 days after completion of study drug, overall success rates (improved or cured) were 116/138 (84.1%) for patients treated with LEVAQUIN ® and 106/132 (80.3%) for patients treated with the comparator.
Success rates varied with the type of diagnosis ranging from 68% in patients with infected ulcers to 90% in patients with infected wounds and abscesses. These rates were equivalent to those seen with comparator drugs.
14.6 Chronic Bacterial Prostatitis
Adult patients with a clinical diagnosis of prostatitis and microbiological culture results from urine sample collected after prostatic massage (VB 3) or expressed prostatic secretion (EPS) specimens obtained via the Meares-Stamey procedure were enrolled in a multicenter, randomized, double-blind study comparing oral LEVAQUIN ® 500 mg, once daily for a total of 28 days to oral ciprofloxacin 500 mg, twice daily for a total of 28 days. The primary efficacy endpoint was microbiologic efficacy in microbiologically evaluable patients. A total of 136 and 125 microbiologically evaluable patients were enrolled in the LEVAQUIN ® and ciprofloxacin groups, respectively. The microbiologic eradication rate by patient infection at 5–18 days after completion of therapy was 75.0% in the LEVAQUIN ® group and 76.8% in the ciprofloxacin group (95% CI [-12.58, 8.98] for LEVAQUIN ® minus ciprofloxacin). The overall eradication rates for pathogens of interest are presented in Table 15.
| LEVAQUIN ® (N = 136) | Ciprofloxacin (N = 125) | |||
|---|---|---|---|---|
| Pathogen | N | Eradication | N | Eradication |
|
|
||||
| E. coli | 15 | 14 (93.3%) | 11 | 9 (81.8%) |
| E. faecalis | 54 | 39 (72.2%) | 44 | 33 (75.0%) |
| S. epidermidis * | 11 | 9 (81.8%) | 14 | 11 (78.6%) |
Eradication rates for S. epidermidis when found with other co-pathogens are consistent with rates seen in pure isolates.
Clinical success (cure + improvement with no need for further antibiotic therapy) rates in microbiologically evaluable population 5–18 days after completion of therapy were 75.0% for LEVAQUIN ®-treated patients and 72.8% for ciprofloxacin-treated patients (95% CI [-8.87, 13.27] for LEVAQUIN ® minus ciprofloxacin). Clinical long-term success (24–45 days after completion of therapy) rates were 66.7% for the LEVAQUIN ®-treated patients and 76.9% for the ciprofloxacin-treated patients (95% CI [-23.40, 2.89] for LEVAQUIN ® minus ciprofloxacin).
14.7 Complicated Urinary Tract Infections and Acute Pyelonephritis: 5-day Treatment Regimen
To evaluate the safety and efficacy of the higher dose and shorter course of LEVAQUIN ®, 1109 patients with cUTI and AP were enrolled in a randomized, double-blind, multicenter clinical trial conducted in the US from November 2004 to April 2006 comparing LEVAQUIN ® 750 mg IV or orally once daily for 5 days (546 patients) with ciprofloxacin 400 mg IV or 500 mg orally twice daily for 10 days (563 patients). Patients with AP complicated by underlying renal diseases or conditions such as complete obstruction, surgery, transplantation, concurrent infection or congenital malformation were excluded. Efficacy was measured by bacteriologic eradication of the baseline organism(s) at the post-therapy visit in patients with a pathogen identified at baseline. The post-therapy (test-of-cure) visit occurred 10 to 14 days after the last active dose of LEVAQUIN ® and 5 to 9 days after the last dose of active ciprofloxacin.
The bacteriologic cure rates overall for LEVAQUIN ® and control at the test-of-cure (TOC) visit for the group of all patients with a documented pathogen at baseline (modified intent to treat or mITT) and the group of patients in the mITT population who closely followed the protocol (Microbiologically Evaluable) are summarized in Table 16.
| LEVAQUIN ® 750 mg orally or IV once daily for 5 days | Ciprofloxacin 400 mg IV/500 mg orally twice daily for 10 days | Overall Difference [95% CI] | |||
|---|---|---|---|---|---|
| n/N | % | n/N | % | LEVAQUIN ®-Ciprofloxacin | |
|
|
|||||
| mITT Population * | |||||
| Overall (cUTI or AP) | 252/333 | 75.7 | 239/318 | 75.2 | 0.5 (-6.1, 7.1) |
| cUTI | 168/230 | 73.0 | 157/213 | 73.7 | |
| AP | 84/103 | 81.6 | 82/105 | 78.1 | |
| Microbiologically Evaluable Population † | |||||
| Overall (cUTI or AP) | 228/265 | 86.0 | 215/241 | 89.2 | -3.2 [-8.9, 2.5] |
| cUTI | 154/185 | 83.2 | 144/165 | 87.3 | |
| AP | 74/80 | 92.5 | 71/76 | 93.4 | |
Microbiologic eradication rates in the Microbiologically Evaluable population at TOC for individual pathogens recovered from patients randomized to LEVAQUIN ® treatment are presented in Table 17.
| Pathogen | Bacteriological Eradication Rate
(n/N) | % |
|---|---|---|
|
|
||
| Escherichia coli * | 155/172 | 90 |
| Klebsiella pneumoniae | 20/23 | 87 |
| Proteus mirabilis | 12/12 | 100 |
14.8 Complicated Urinary Tract Infections and Acute Pyelonephritis: 10-day Treatment Regimen
To evaluate the safety and efficacy of the 250 mg dose, 10 day regimen of LEVAQUIN ®, 567 patients with uncomplicated UTI, mild-to-moderate cUTI, and mild-to-moderate AP were enrolled in a randomized, double-blind, multicenter clinical trial conducted in the US from June 1993 to January 1995 comparing LEVAQUIN ® 250 mg orally once daily for 10 days (285 patients) with ciprofloxacin 500 mg orally twice daily for 10 days (282 patients). Patients with a resistant pathogen, recurrent UTI, women over age 55 years, and with an indwelling catheter were initially excluded, prior to protocol amendment which took place after 30% of enrollment. Microbiological efficacy was measured by bacteriologic eradication of the baseline organism(s) at 1–12 days post-therapy in patients with a pathogen identified at baseline.
The bacteriologic cure rates overall for LEVAQUIN ® and control at the test-of-cure (TOC) visit for the group of all patients with a documented pathogen at baseline (modified intent to treat or mITT) and the group of patients in the mITT population who closely followed the protocol (Microbiologically Evaluable) are summarized in Table 18.
| LEVAQUIN
®
250 mg once daily for 10 days | Ciprofloxacin
500 mg twice daily for 10 days |
|||
|---|---|---|---|---|
| n/N | % | n/N | % | |
|
|
||||
| mITT Population † | 174/209 | 83.3 | 184/219 | 84.0 |
| Microbiologically Evaluable Population ‡ | 164/177 | 92.7 | 159/171 | 93.0 |
14.9 Inhalational Anthrax (Post-Exposure)
The effectiveness of LEVAQUIN ® for this indication is based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit. LEVAQUIN ® has not been tested in humans for the post-exposure prevention of inhalation anthrax. The mean plasma concentrations of LEVAQUIN ® associated with a statistically significant improvement in survival over placebo in the rhesus monkey model of inhalational anthrax are reached or exceeded in adult and pediatric patients receiving the recommended oral and intravenous dosage regimens [see Indications and Usage (1.13) and Dosage and Administration (2.1, 2.2)] .
Levofloxacin pharmacokinetics have been evaluated in adult and pediatric patients. The mean (± SD) steady state peak plasma concentration in human adults receiving 500 mg orally or intravenously once daily is 5.7 ± 1.4 and 6.4 ± 0.8 mcg/mL, respectively; and the corresponding total plasma exposure (AUC 0–24) is 47.5 ± 6.7 and 54.6 ± 11.1 mcg.h/mL, respectively. The predicted steady-state pharmacokinetic parameters in pediatric patients ranging in age from 6 months to 17 years receiving 8 mg/kg orally every 12 hours (not to exceed 250 mg per dose) were calculated to be comparable to those observed in adults receiving 500 mg orally once daily [see Clinical Pharmacology (12.3)] . LEVAQUIN ® Tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)].
In adults, the safety of LEVAQUIN ® for treatment durations of up to 28 days is well characterized. However, information pertaining to extended use at 500 mg daily up to 60 days is limited. Prolonged LEVAQUIN ® therapy in adults should only be used when the benefit outweighs the risk.
In pediatric patients, the safety of levofloxacin for treatment durations of more than 14 days has not been studied. An increased incidence of musculoskeletal adverse events (arthralgia, arthritis, tendinopathy, gait abnormality) compared to controls has been observed in clinical studies with treatment duration of up to 14 days. Long-term safety data, including effects on cartilage, following the administration of levofloxacin to pediatric patients is limited [see Warnings and Precautions (5.10) and Use in Specific Populations (8.4)] .
A placebo-controlled animal study in rhesus monkeys exposed to an inhaled mean dose of 49 LD 50 (~2.7 × 10 6) spores (range 17 – 118 LD 50) of B. anthracis (Ames strain) was conducted. The minimal inhibitory concentration (MIC) of levofloxacin for the anthrax strain used in this study was 0.125 mcg/mL. In the animals studied, mean plasma concentrations of levofloxacin achieved at expected T max (1 hour post-dose) following oral dosing to steady state ranged from 2.79 to 4.87 mcg/mL. Steady state trough concentrations at 24 hours post-dose ranged from 0.107 to 0.164 mcg/mL. Mean (SD) steady state AUC 0–24 was 33.4 ± 3.2 mcg.h/mL (range 30.4 to 36.0 mcg.h/mL). Mortality due to anthrax for animals that received a 30 day regimen of oral LEVAQUIN ® beginning 24 hrs post exposure was significantly lower (1/10), compared to the placebo group (9/10) [P = 0.0011, 2-sided Fisher's Exact Test]. The one levofloxacin treated animal that died of anthrax did so following the 30-day drug administration period.
14.10 Plague
Efficacy studies of LEVAQUIN ® could not be conducted in humans with pneumonic plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals.
The mean plasma concentrations of LEVAQUIN ® associated with a statistically significant improvement in survival over placebo in an African green monkey model of pneumonic plague are reached or exceeded in adult and pediatric patients receiving the recommended oral and intravenous dosage regimens [see Indications and Usage (1.14) and Dosage and Administration (2.1), (2.2)] .
Levofloxacin pharmacokinetics have been evaluated in adult and pediatric patients. The mean (± SD) steady state peak plasma concentration in human adults receiving 500 mg orally or intravenously once daily is 5.7 ± 1.4 and 6.4 ± 0.8 mcg/mL, respectively; and the corresponding total plasma exposure (AUC 0–24) is 47.5 ± 6.7 and 54.6 ± 11.1 mcg.h/mL, respectively. The predicted steady-state pharmacokinetic parameters in pediatric patients ranging in age from 6 months to 17 years receiving 8 mg/kg orally every 12 hours (not to exceed 250 mg per dose) were calculated to be comparable to those observed in adults receiving 500 mg orally once daily [see Clinical Pharmacology (12.3)] . LEVAQUIN ® Tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)].
A placebo-controlled animal study in African green monkeys exposed to an inhaled mean dose of 65 LD 50 (range 3 to 145 LD 50) of Yersinia pestis (CO92 strain) was conducted. The minimal inhibitory concentration (MIC) of levofloxacin for the Y. pestis strain used in this study was 0.03 mcg/mL. Mean plasma concentrations of levofloxacin achieved at the end of a single 30-min infusion ranged from 2.84 to 3.50 mcg/mL in African green monkeys. Trough concentrations at 24 hours post-dose ranged from <0.03 to 0.06 mcg/mL. Mean (SD) AUC 0–24 was 11.9 (3.1) mcg.h/mL (range 9.50 to 16.86 mcg.h/mL). Animals were randomized to receive either a 10-day regimen of i.v. LEVAQUIN ® or placebo beginning within 6 hrs of the onset of telemetered fever (≥ 39°C for more than 1 hour). Mortality in the LEVAQUIN ® group was significantly lower (1/17) compared to the placebo group (7/7) [p<0.001, Fisher's Exact Test; exact 95% confidence interval (-99.9%, -55.5%) for the difference in mortality]. One levofloxacin-treated animal was euthanized on Day 9 post-exposure to Y. pestis due to a gastric complication; it had a blood culture positive for Y. pestis on Day 3 and all subsequent daily blood cultures from Day 4 through Day 7 were negative.
16 HOW SUPPLIED/STORAGE AND HANDLING
LEVAQUIN ® Tablets are supplied as 250, 500, and 750 mg capsule-shaped, coated tablets. LEVAQUIN ® Tablets are packaged in bottles in the following configurations:
- 250 mg tablets are terra cotta pink and are imprinted: "LEVAQUIN" on one side and "250" on the other side
- bottles of 50 (NDC: 50458-920-50)
- 500 mg tablets are peach and are imprinted: "LEVAQUIN" on one side and "500" on the other side
- bottles of 50 (NDC: 50458-925-50)
- 750 mg tablets are white and are imprinted "LEVAQUIN" on one side and "750" on the other side
- bottles of 20 (NDC: 50458-930-20)
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Serious Adverse Reactions
Advise patients to stop taking LEVAQUIN ® if they experience an adverse reaction and to call their healthcare provider for advice on completing the full course of treatment with another antibacterial drug.
Inform patients of the following serious adverse reactions that have been associated with LEVAQUIN ® or other fluoroquinolone use:
- Disabling and Potentially Irreversible Serious Adverse Reactions That May Occur Together: Inform patients that disabling and potentially irreversible serious adverse reactions, including tendinitis and tendon rupture, peripheral neuropathies, and central nervous system effects, have been associated with use of LEVAQUIN ® and may occur together in the same patient. Inform patients to stop taking LEVAQUIN ® immediately if they experience an adverse reaction and to call their healthcare provider.
- Tendinitis and Tendon Rupture: Instruct patients to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue LEVAQUIN ® treatment. Symptoms may be irreversible. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Peripheral Neuropathies: Inform patients that peripheral neuropathies have been associated with levofloxacin use, symptoms may occur soon after initiation of therapy and may be irreversible. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, immediately discontinue LEVAQUIN ® and tell them to contact their physician.
- Central Nervous System Effects (for example, convulsions, dizziness, lightheadedness, increased intracranial pressure) : Inform patients that convulsions have been reported in patients receiving fluoroquinolones, including levofloxacin. Instruct patients to notify their physician before taking this drug if they have a history of convulsions. Inform patients that they should know how they react to LEVAQUIN ® before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination. Instruct patients to notify their physician if persistent headache with or without blurred vision occurs.
- Exacerbation of Myasthenia Gravis: Instruct patients to inform their physician of any history of myasthenia gravis. Instruct patients to notify their physician if they experience any symptoms of muscle weakness, including respiratory difficulties.
- Hypersensitivity Reactions: Inform patients that levofloxacin can cause hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (for example, swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction.
- Hepatotoxicity: Inform patients that severe hepatotoxicity (including acute hepatitis and fatal events) has been reported in patients taking LEVAQUIN ®. Instruct patients to inform their physician if they experience any signs or symptoms of liver injury including: loss of appetite, nausea, vomiting, fever, weakness, tiredness, right upper quadrant tenderness, itching, yellowing of the skin and eyes, light colored bowel movements or dark colored urine.
- Diarrhea: Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, instruct patients to contact their physician as soon as possible.
- Prolongation of the QT Interval: Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Instruct patients to notify their physician if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness.
- Musculoskeletal Disorders in Pediatric Patients: Instruct parents to inform their child's physician if the child has a history of joint-related problems before taking this drug. Inform parents of pediatric patients to notify their child's physician of any joint-related problems that occur during or following levofloxacin therapy [see Warnings and Precautions (5.11) and Use in Specific Populations (8.4)].
- Photosensitivity/Phototoxicity: Inform patients that photosensitivity/phototoxicity has been reported in patients receiving fluoroquinolones. Inform patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking fluoroquinolones. If patients need to be outdoors while using fluoroquinolones, instruct them to wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, instruct patients to contact their physician.
Antibacterial Resistance
Antibacterial drugs including LEVAQUIN ® should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When LEVAQUIN ® is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by LEVAQUIN ® or other antibacterial drugs in the future.
Administration with Food, Fluids, and Concomitant Medications
Patients should be informed that LEVAQUIN ® Tablets may be taken with or without food. The tablets should be taken at the same time each day.
Patients should drink fluids liberally while taking LEVAQUIN ® to avoid formation of a highly concentrated urine and crystal formation in the urine.
Antacids containing magnesium, or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine should be taken at least two hours before or two hours after oral LEVAQUIN ® administration.
Drug Interactions with Insulin, Oral Hypoglycemic Agents, and Warfarin
Patients should be informed that if they are diabetic and are being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, they should discontinue LEVAQUIN ® and consult a physician.
Patients should be informed that concurrent administration of warfarin and LEVAQUIN ® has been associated with increases of the International Normalized Ratio (INR) or prothrombin time and clinical episodes of bleeding. Patients should notify their physician if they are taking warfarin, be monitored for evidence of bleeding, and also have their anticoagulation tests closely monitored while taking warfarin concomitantly.
Active Ingredient Made in Japan
LEVAQUIN ® Tablets are Manufactured by:
Janssen Ortho LLC, Gurabo, Puerto Rico 00778
Manufactured for:
Janssen Pharmaceuticals, Inc., Titusville, NJ 08560
© 1996, 2007 Janssen Pharmaceutical Companies
| This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 07/2018 | |||
| MEDICATION GUIDE
LEVAQUIN ® (Leave ah kwin) (levofloxacin) tablets |
|||
|
What is the most important information I should know about LEVAQUIN? LEVAQUIN, a fluoroquinolone antibiotic, can cause serious side effects. Some of these serious side effects can happen at the same time and could result in death. If you have any of the following serious side effects while you take LEVAQUIN, you should stop taking LEVAQUIN immediately and get medical help right away.
|
|||
|
|
||
The nerve damage may be permanent.
|
|||
|
|
||
|
|||
|
What is LEVAQUIN? LEVAQUIN is a fluoroquinolone antibiotic medicine used in adults age 18 years or older to treat certain infections caused by certain germs called bacteria. These bacterial infections include: |
|||
|
|
||
|
Studies of LEVAQUIN for use in the treatment of plague and anthrax were done in animals only, because plague and anthrax could not be studied in people. LEVAQUIN should not be used in patients with uncomplicated urinary tract infections, acute bacterial exacerbation of chronic bronchitis, or acute bacterial sinusitis if there are other treatment options available. LEVAQUIN Tablets is also used to treat children who weigh at least 66 pounds (or at least 30 kilograms) and may have breathed in anthrax germs, have plague, or been exposed to plague germs. It is not known if LEVAQUIN is safe and effective in children under 6 months of age. The safety and effectiveness in children treated with LEVAQUIN for more than 14 days is not known. |
|||
|
Who should not take LEVAQUIN? Do not take LEVAQUIN: if you have ever had a severe allergic reaction to an antibiotic known as a fluoroquinolone, or if you are allergic to levofloxacin or any of the ingredients in LEVAQUIN. See the end of this leaflet for a complete list of ingredients in LEVAQUIN. |
|||
|
Before you take LEVAQUIN, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEVAQUIN and other medicines can affect each other causing side effects. Especially tell your healthcare provider if you take:
Ask your healthcare provider if you are not sure if any of your medicines are listed above. Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. |
|||
|
How should I take LEVAQUIN?
Taking all of your LEVAQUIN doses will help make sure that all of the bacteria are killed. Taking all of your LEVAQUIN doses will help you lower the chance that the bacteria will become resistant to LEVAQUIN. If your infection does not get better while you take LEVAQUIN, it may mean that the bacteria causing your infection may be resistant to LEVAQUIN. If your infection does not get better, call your healthcare provider. If your infection does not get better, LEVAQUIN and other similar antibiotic medicines may not work for you in the future.
|
|||
|
What should I avoid while taking LEVAQUIN?
|
|||
|
What are the possible side effects of LEVAQUIN? LEVAQUIN may cause serious side effects, including:
Allergic reactions can happen in people taking fluoroquinolones, including LEVAQUIN, even after only 1 dose. Stop taking LEVAQUIN and get emergency medical help right away if you have any of the following symptoms of a severe allergic reaction:
Skin rash may happen in people taking LEVAQUIN, even after only 1 dose. Stop taking LEVAQUIN at the first sign of a skin rash and immediately call your healthcare provider. Skin rash may be a sign of a more serious reaction to LEVAQUIN.
Stop taking LEVAQUIN and tell your healthcare provider right away if you have yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to LEVAQUIN (a liver problem).
In children 6 months and older who take LEVAQUIN to treat anthrax disease or plague, vomiting is also common. LEVAQUIN may cause false-positive urine screening results for opiates when testing is done with some commercially available kits. A positive result should be confirmed using a more specific test. These are not all the possible side effects of LEVAQUIN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
How should I store LEVAQUIN?
|
|||
|
General information about the safe and effective use of LEVAQUIN. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LEVAQUIN for a condition for which it is not prescribed. Do not give LEVAQUIN to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about LEVAQUIN. If you would like more information about LEVAQUIN, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about LEVAQUIN that is written for healthcare professionals. |
|||
|
What are the ingredients in LEVAQUIN? Active ingredient: levofloxacin Inactive ingredients: crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide. LEVAQUIN 250 mg Tablets also contain synthetic red iron oxide. LEVAQUIN 500 mg Tablets also contain synthetic red iron oxide and synthetic yellow iron oxide. Manufactured by:
© 1996, 2007 Janssen Pharmaceutical Companies For more information, go to www.levaquin.com or call 1-800-526-7736 |
|||
DRUG: Levaquin
GENERIC: levofloxacin
DOSAGE: TABLET, FILM COATED
ADMINSTRATION: ORAL
NDC: 70518-0501-0
COLOR: white
SHAPE: OVAL
SCORE: No score
SIZE: 20 mm
IMPRINT: LEVAQUIN;750
PACKAGING: 10 in 1 BOTTLE, PLASTIC
ACTIVE INGREDIENT(S):
- levofloxacin 750mg in 1
INACTIVE INGREDIENT(S):
- hypromellose, unspecified
- polyethylene glycol, unspecified
- titanium dioxide
- magnesium stearate
- crospovidone (15 mpa.s at 5%)
- microcrystalline cellulose
- polysorbate 80

| LEVAQUIN
levofloxacin tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |
Trademark Results [Levaquin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LEVAQUIN 77583537 not registered Dead/Abandoned |
DAIICHI SANKYO COMPANY, LIMITED 2008-10-01 |
 LEVAQUIN 75008829 2078038 Live/Registered |
DAIICHI SANKYO COMPANY, LIMITED 1995-10-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.