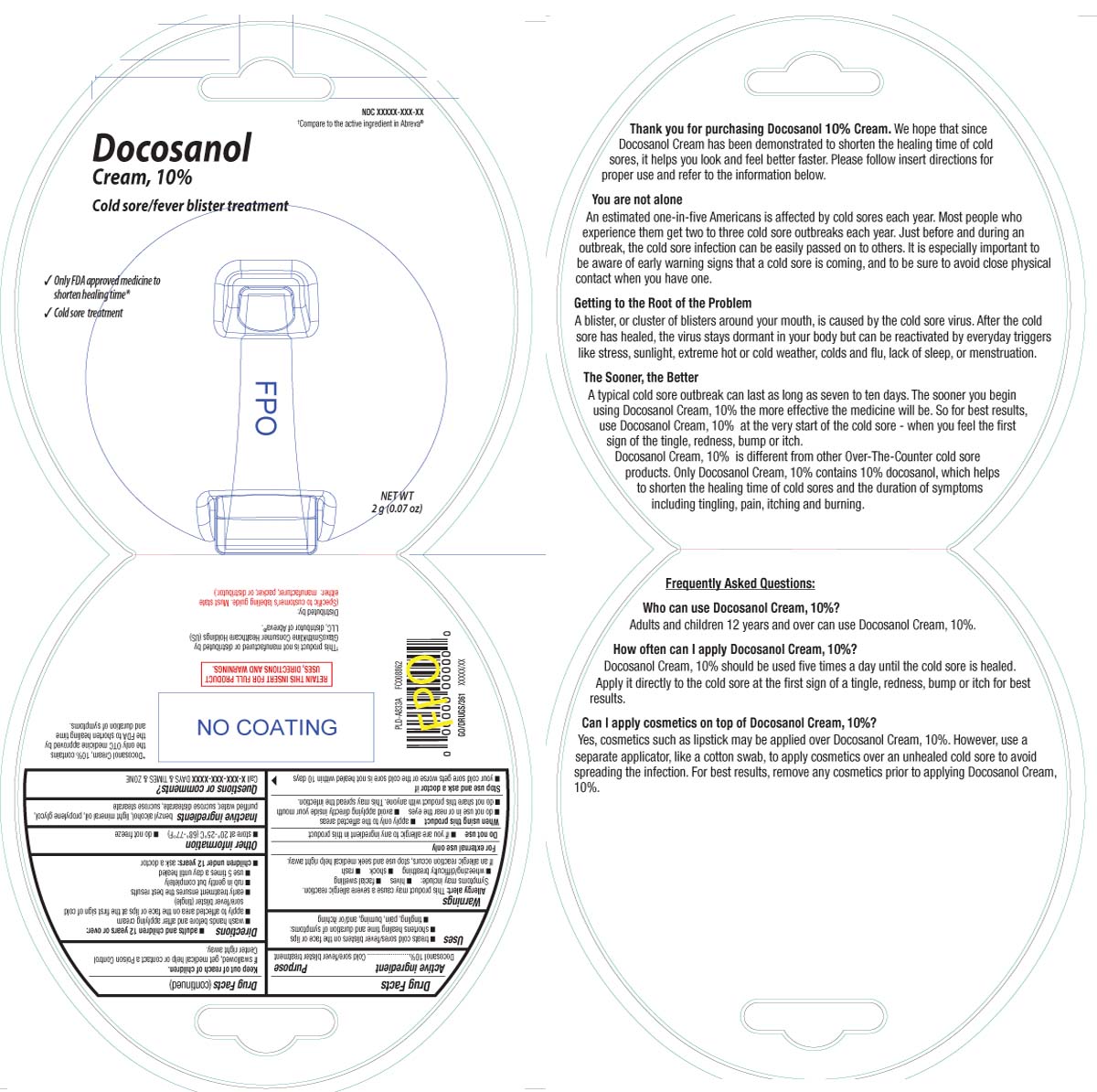
COLD SORE FEVER BLISTER TREATMENT- docosanol cream
Cold Sore Fever Blister Treatment by
Drug Labeling and Warnings
Cold Sore Fever Blister Treatment by is a Otc medication manufactured, distributed, or labeled by Topco Associates. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
-
adults and children 12 years or over:
- wash hands before and after applying cream
- apply to affected area on the face or lips at the first sign of cold sore/fever blister (tingle).
- early treatment ensures the best results
- rub in gently but completely
- use 5 times a day until healed
- children under 12 years: ask a doctor
-
adults and children 12 years or over:
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
†COMPARE TO THE ACTIVE INGREDIENT IN ABREVA®
Cold Sore Treatment
COLD SORE/FEVER BLISTER TREATMENT
DOCOSANOL CREAM, 10%
- Only FDA Approved Medicine to Shorten Healing Time*
- Cold Sore Treatment
NET WT 2 g (0.07 oz)
*Docosanol Cream, 10% contains the only OTC medicine approved by the FDA to shorten healing time and duration of symptoms.
†This product is not manufactured or distributed by GlaxoSmithKline Consumer Healthcare Holdings (US) LLC, distributor of Abreva®.
RETAIN THIS INSERT FOR FULL PRODUCT USES, DIRECTIONS AND WARNINGS
DISTRIBUTED BY
TOPCO ASSOCIATES LLC
ITASCA, IL 60143
- Package Label
-
INGREDIENTS AND APPEARANCE
COLD SORE FEVER BLISTER TREATMENT
docosanol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76162-818 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCOSANOL (UNII: 9G1OE216XY) (DOCOSANOL - UNII:9G1OE216XY) DOCOSANOL 100 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCROSE DISTEARATE (HLB 5) (UNII: 33X4X4B90S) SUCROSE STEARATE (UNII: 274KW0O50M) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76162-818-07 1 in 1 PACKAGE 12/31/2023 06/30/2028 1 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212385 12/31/2023 06/30/2028 Labeler - Topco Associates (006935977)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.