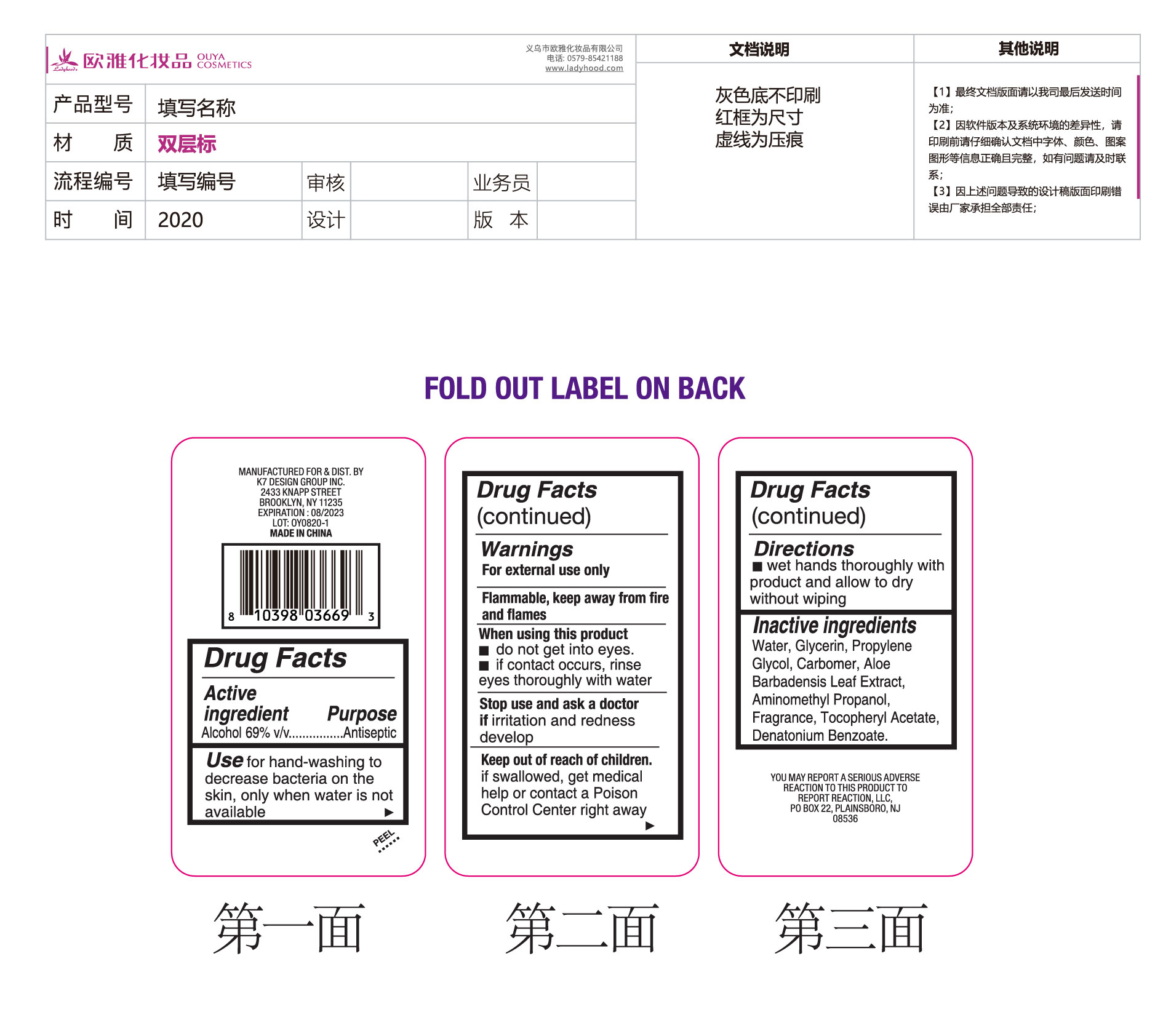
ULTRA DEFENSE SANI SMART HAND SANITIZER LAVENDER SCENT WITH VITAMIN E by Ouya Cosmetics Co., Ltd of Yiwu
ULTRA DEFENSE SANI SMART HAND SANITIZER LAVENDER SCENT WITH VITAMIN E by
Drug Labeling and Warnings
ULTRA DEFENSE SANI SMART HAND SANITIZER LAVENDER SCENT WITH VITAMIN E by is a Otc medication manufactured, distributed, or labeled by Ouya Cosmetics Co., Ltd of Yiwu. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ULTRA DEFENSE SANI SMART HAND SANITIZER LAVENDER SCENT WITH VITAMIN E- alcohol solution
Ouya Cosmetics Co., Ltd of Yiwu
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| ULTRA DEFENSE SANI SMART HAND SANITIZER LAVENDER SCENT WITH VITAMIN E
alcohol solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Ouya Cosmetics Co., Ltd of Yiwu (546464926) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ouya Cosmetics Co., Ltd of Yiwu | 546464926 | manufacture(54699-006) | |
Revised: 7/2020
Document Id: aa87567c-a08d-a13f-e053-2995a90a7a28
Set id: 73522323-5c7c-467d-aed5-ef9d0729074a
Version: 1
Effective Time: 20200715