TNKASE- tenecteplase kit
TNKase by
Drug Labeling and Warnings
TNKase by is a Prescription medication manufactured, distributed, or labeled by Genentech, Inc., Genentech, Inc. (Hillsboro), Genentech, Inc. (OCN). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
TNKase® (Tenecteplase) is a tissue plasminogen activator (tPA) produced by recombinant DNA technology using an established mammalian cell line (Chinese Hamster Ovary cells). Tenecteplase is a 527 amino acid glycoprotein developed by introducing the following modifications to the complementary DNA (cDNA) for natural human tPA: a substitution of threonine 103 with asparagine, and a substitution of asparagine 117 with glutamine, both within the kringle 1 domain, and a tetra-alanine substitution at amino acids 296–299 in the protease domain. TNKase is a sterile, white to off-white, lyophilized powder for single intravenous (IV) bolus administration after reconstitution with Sterile Water for Injection (SWFI), USP. Each vial of TNKase nominally contains 52.5 mg Tenecteplase, 0.55 g L-arginine, 0.17 g phosphoric acid, and 4.3 mg polysorbate 20, which includes a 5% overfill. Each vial will deliver 50 mg of Tenecteplase.
-
CLINICAL PHARMACOLOGY
General
Tenecteplase is a modified form of human tissue plasminogen activator (tPA) that binds to fibrin and converts plasminogen to plasmin. In the presence of fibrin, in vitro studies demonstrate that Tenecteplase conversion of plasminogen to plasmin is increased relative to its conversion in the absence of fibrin. This fibrin specificity decreases systemic activation of plasminogen and the resulting degradation of circulating fibrinogen as compared to a molecule lacking this property. Following administration of 30, 40, or 50 mg of TNKase, there are decreases in circulating fibrinogen (4%–15%) and plasminogen (11%–24%). The clinical significance of fibrin-specificity on safety (e.g., bleeding) or efficacy has not been established. Biological potency is determined by an in vitro clot lysis assay and is expressed in Tenecteplase-specific units. The specific activity of Tenecteplase has been defined as 200 units/mg.
Pharmacokinetics
In patients with acute myocardial infarction (AMI), TNKase administered as a single bolus exhibits a biphasic disposition from the plasma. Tenecteplase was cleared from the plasma with an initial half-life of 20 to 24 minutes. The terminal phase half-life of Tenecteplase was 90 to 130 minutes. In 99 of 104 patients treated with Tenecteplase, mean plasma clearance ranged from 99 to 119 mL/min.
The initial volume of distribution is weight related and approximates plasma volume. Liver metabolism is the major clearance mechanism for Tenecteplase.
-
CLINICAL STUDIES
ASSENT-2 was an international, randomized, double-blind trial that compared 30-day mortality rates in 16,949 patients assigned to receive an IV bolus dose of TNKase or an accelerated infusion of Activase® (Alteplase).1 Eligibility criteria included onset of chest pain within 6 hours of randomization and ST-segment elevation or left bundle branch block on electrocardiogram (ECG). Patients were to be excluded from the trial if they received GP IIb/IIIa inhibitors within the previous 12 hours. TNKase was dosed using actual or estimated weight in a weight-tiered fashion as described in DOSAGE AND ADMINISTRATION. All patients were to receive 150–325 mg of aspirin administered as soon as possible, followed by 150–325 mg daily. Intravenous heparin was to be administered as soon as possible: for patients weighing ≤67 kg, heparin was administered as a 4000 unit IV bolus followed by infusion at 800 U/hr; for patients weighing >67 kg, heparin was administered as a 5000 unit IV bolus followed by infusion at 1000 U/hr. Heparin was continued for 48 to 72 hours with infusion adjusted to maintain aPTT at 50–75 seconds. The use of GP IIb/IIIa inhibitors was discouraged for the first 24 hours following randomization. The results of the primary endpoint (30-day mortality rates with non-parametric adjustment for the covariates of age, Killip class, heart rate, systolic blood pressure and infarct location) along with selected other 30-day endpoints are shown in Table 1.
Table 1 ASSENT-2 Mortality, Stroke, and Combined Outcome of Death or Stroke Measured at Thirty Days 30-Day Events TNKase
(n = 8461)Accelerated Activase
(n = 8488)Relative Risk TNKase/Activase
(95% CI)Mortality 6.2% 6.2% 1.00
(0.89, 1.12)Intracranial Hemorrhage (ICH) 0.9% 0.9% 0.99
(0.73, 1.35)Any Stroke 1.8% 1.7% 1.07
(0.86, 1.35)Death or Nonfatal Stroke 7.1% 7.0% 1.01
(0.91, 1.13)Rates of mortality and the combined endpoint of death or stroke among pre-specified subgroups, including age, gender, time to treatment, infarct location, and history of previous myocardial infarction, demonstrate consistent relative risks across these subgroups. There was insufficient enrollment of non-Caucasian patients to draw any conclusions regarding relative efficacy in racial subsets.
Rates of in-hospital procedures, including percutaneous transluminal coronary angioplasty (PTCA), stent placement, intra-aortic balloon pump (IABP) use, and coronary artery bypass graft (CABG) surgery, were similar between the TNKase and Activase®(Alteplase) groups.
TIMI 10B was an open-label, controlled, randomized, dose-ranging, angiography study which utilized a blinded core laboratory for review of coronary arteriograms.2 Patients (n = 837) presenting within 12 hours of symptom onset were treated with fixed doses of 30, 40, or 50 mg of TNKase or the accelerated infusion of Activase and underwent coronary arteriography at 90 minutes. The results showed that the 40 mg and 50 mg doses were similar to accelerated infusion of Activase in restoring patency. TIMI Grade 3 flow and TIMI Grade 2/3 flow at 90 minutes are shown in Table 2. The exact relationship between coronary artery patency and clinical activity has not been established.
Table 2 TIMI 10B Patency Rates TIMI Grade Flow at 90 Minutes Activase ≤100 mg
(n=311)TNKase 30 mg
(n=302)TNKase 40 mg
(n=148)TNKase 50 mg
(n=76)TIMI Grade 3 Flow 63% 54% 63% 66% TIMI Grade 2/3 Flow 82% 77% 79% 88% 95% CI
(TIMI 2/3 Flow)(77%,86%) (72%,81%) (72%,85%) (79%,94%) The angiographic results from TIMI 10B and the safety data from ASSENT-1, an additional uncontrolled safety study of 3,235 TNKase-treated patients, provided the framework to develop a weight-tiered TNKase dose regimen.3 Exploratory analyses suggested that a weight-adjusted dose of 0.5 mg/kg to 0.6 mg/kg of TNKase resulted in a better patency to bleeding relationship than fixed doses of TNKase across a broad range of patient weights.
The Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT 4 PCI) was a Phase IIIb/IV study designed to assess the safety and effectiveness of a strategy of administering full dose TNKase with a single bolus of 4000 U of unfractionated heparin in patients with ST segment elevation AMI, in whom primary percutaneous coronary intervention (PCI) was planned, but in whom a delay of 1–3 hours was anticipated before PCI. The trial was prematurely terminated with 1667 randomized patients (75 of whom were treated in the United States) due to a numerically higher mortality in the patients receiving TNKase prior to primary PCI versus PCI without TNKase (median time from randomization to balloon of 115 minutes). The incidence of the 90‑day primary endpoint, a composite of death or cardiogenic shock or congestive heart failure (CHF) within 90 days, was 18.6% in patients treated with TNKase plus PCI versus 13.4% in those treated with PCI alone (p=0.0055; OR 1.39 (95% C.I. 1.11–1.74)).
There were trends toward worse outcomes in the individual components of the primary endpoint between TNKase plus PCI versus PCI alone (mortality 6.7% vs. 5.0%, respectively; cardiogenic shock 6.1% vs. 4.8%, respectively; and CHF 12.1% vs. 9.4%, respectively). In addition, there were trends towards worse outcomes in recurrent MI (6.1% vs. 3.5%, respectively; p=0.03) and repeat target vessel revascularization (6.6% vs. 3.6%, respectively; p=0.005) in patients receiving TNKase plus PCI versus PCI alone.
There was no difference in in‑hospital major bleeding between the two groups (5.6% vs. 4.4% for TNKase plus PCI vs. PCI alone, respectively). For patients treated with TNKase plus PCI, in‑hospital rates of intracranial hemorrhage and total stroke were similar to those observed in previous trials (0.97% and 1.8%, respectively); however, none of the patients treated with PCI alone experienced a stroke (ischemic, hemorrhagic or other).
-
INDICATIONS AND USAGE
TNKase® (Tenecteplase) is indicated for use in the reduction of mortality associated with acute myocardial infarction (AMI). Treatment should be initiated as soon as possible after the onset of AMI symptoms (see CLINICAL STUDIES).
-
CONTRAINDICATIONS
TNKase therapy in patients with acute myocardial infarction is contraindicated in the following situations because of an increased risk of bleeding (see WARNINGS):
- Active internal bleeding
- History of cerebrovascular accident
- Intracranial or intraspinal surgery or trauma within 2 months
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Known bleeding diathesis
- Severe uncontrolled hypertension
-
WARNINGS
Bleeding
The most common complication encountered during TNKase therapy is bleeding. The type of bleeding associated with thrombolytic therapy can be divided into two broad categories:
- Internal bleeding, involving intracranial and retroperitoneal sites, or the gastrointestinal, genitourinary, or respiratory tracts.
- Superficial or surface bleeding, observed mainly at vascular puncture and access sites (e.g., venous cutdowns, arterial punctures) or sites of recent surgical intervention.
Should serious bleeding (not controlled by local pressure) occur, any concomitant heparin or antiplatelet agents should be discontinued immediately and treated appropriately.
In clinical studies of TNKase, patients were treated with both aspirin and heparin. Heparin may contribute to the bleeding risks associated with TNKase. The safety of the use of TNKase with other antiplatelet agents has not been adequately studied (see PRECAUTIONS: Drug Interactions). Intramuscular injections and nonessential handling of the patient should be avoided for the first few hours following treatment with TNKase. Venipunctures should be performed and monitored carefully.
Should an arterial puncture be necessary during the first few hours following TNKase therapy, it is preferable to use an upper extremity vessel that is accessible to manual compression. Pressure should be applied for at least 30 minutes, a pressure dressing applied, and the puncture site checked frequently for evidence of bleeding.
Each patient being considered for therapy with TNKase should be carefully evaluated and anticipated benefits weighed against potential risks associated with therapy. In the following conditions, the risk of TNKase therapy may be increased and should be weighed against the anticipated benefits:
- Recent major surgery, e.g., coronary artery bypass graft, obstetrical delivery, organ biopsy, previous puncture of noncompressible vessels
- Cerebrovascular disease
- Recent gastrointestinal or genitourinary bleeding
- Recent trauma
- Hypertension: systolic BP ≥180 mm Hg and/or diastolic BP ≥110 mm Hg
- Acute pericarditis
- Subacute bacterial endocarditis
- Hemostatic defects, including those secondary to severe hepatic or renal disease
- Severe hepatic dysfunction
- Pregnancy
- Diabetic hemorrhagic retinopathy or other hemorrhagic ophthalmic conditions
- Septic thrombophlebitis or occluded AV cannula at seriously infected site
- Advanced age (see PRECAUTIONS: Geriatric Use)
- Patients currently receiving oral anticoagulants, e.g., warfarin sodium
- Recent administration of GP IIb/IIIa inhibitors
- Any other condition in which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location
Thromboembolism
The use of thrombolytics can increase the risk of thrombo-embolic events in patients with high likelihood of left heart thrombus, such as patients with mitral stenosis or atrial fibrillation.
Cholesterol Embolization
Cholesterol embolism has been reported rarely in patients treated with all types of thrombolytic agents; the true incidence is unknown. This serious condition, which can be lethal, is also associated with invasive vascular procedures (e.g., cardiac catheterization, angiography, vascular surgery) and/or anticoagulant therapy. Clinical features of cholesterol embolism may include livedo reticularis, "purple toe" syndrome, acute renal failure, gangrenous digits, hypertension, pancreatitis, myocardial infarction, cerebral infarction, spinal cord infarction, retinal artery occlusion, bowel infarction, and rhabdomyolysis.
Arrhythmias
Coronary thrombolysis may result in arrhythmias associated with reperfusion. These arrhythmias (such as sinus bradycardia, accelerated idioventricular rhythm, ventricular premature depolarizations, ventricular tachycardia) are not different from those often seen in the ordinary course of acute myocardial infarction and may be managed with standard anti‑arrhythmic measures. It is recommended that anti‑arrhythmic therapy for bradycardia and/or ventricular irritability be available when TNKase is administered.
Use with Percutaneous Coronary Intervention (PCI)
In patients with large ST segment elevation myocardial infarction, physicians should choose either thrombolysis or PCI as the primary treatment strategy for reperfusion. Rescue PCI or subsequent elective PCI may be performed after administration of thrombolytic therapies if medically appropriate; however, the optimal use of adjunctive antithrombotic and antiplatelet therapies in this setting is unknown.
-
PRECAUTIONS
General
Standard management of myocardial infarction should be implemented concomitantly with TNKase treatment. Arterial and venous punctures should be minimized. Noncompressible arterial puncture must be avoided and internal jugular and subclavian venous punctures should be avoided to minimize bleeding from the noncompressible sites. In the event of serious bleeding, heparin and antiplatelet agents should be discontinued immediately. Heparin effects can be reversed by protamine.
Readministration
Readministration of plasminogen activators, including TNKase, to patients who have received prior plasminogen activator therapy has not been systematically studied. Three of 487 patients tested for antibody formation to TNKase had a positive antibody titer at 30 days. The data reflect the percentage of patients whose test results were considered positive for antibodies to TNKase in a radioimmunoprecipitation assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to TNKase with the incidence of antibodies to other products may be misleading. Although sustained antibody formation in patients receiving one dose of TNKase has not been documented, readministration should be undertaken with caution.
Hypersensitivity
Hypersensitivity, including urticarial / anaphylactic reactions, have been reported after administration of TNKase (e.g., anaphylaxis, angioedema, laryngeal edema, rash, and urticaria). Monitor patients treated with TNKase during and for several hours after infusion. If symptoms of hypersensitivity occur, appropriate therapy should be initiated.
Drug Interactions
Formal interaction studies of TNKase with other drugs have not been performed. Patients studied in clinical trials of TNKase were routinely treated with heparin and aspirin. Anticoagulants (such as heparin and vitamin K antagonists) and drugs that alter platelet function (such as acetylsalicylic acid, dipyridamole, and GP IIb/IIIa inhibitors) may increase the risk of bleeding if administered prior to, during, or after TNKase therapy.
Drug/Laboratory Test Interactions
During TNKase therapy, results of coagulation tests and/or measures of fibrinolytic activity may be unreliable unless specific precautions are taken to prevent in vitro artifacts. Tenecteplase is an enzyme that, when present in blood in pharmacologic concentrations, remains active under in vitro conditions. This can lead to degradation of fibrinogen in blood samples removed for analysis.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed to evaluate the carcinogenic potential, mutagenicity, or the effect on fertility.
Pregnancy
TNKase has been shown to elicit maternal and embryo toxicity in rabbits given multiple IV administrations. In rabbits administered 0.5, 1.5, and 5.0 mg/kg/day during organogenesis, vaginal hemorrhage resulted in maternal deaths. Subsequent embryonic deaths were secondary to maternal hemorrhage and no fetal anomalies were observed. TNKase does not elicit maternal and embryo toxicity in rabbits following a single IV administration. Thus, in developmental toxicity studies conducted in rabbits, the no observable effect level (NOEL) of a single IV administration of TNKase on maternal or developmental toxicity (5 mg/kg) was approximately 7 times human exposure (based on AUC) at the dose for AMI. There are no adequate and well-controlled studies in pregnant women. TNKase should be given to pregnant women only if the potential benefits justify the potential risk to the fetus.
Nursing Mothers
It is not known if TNKase is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TNKase is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of TNKase in pediatric patients have not been established.
Geriatric Use
Of the patients in ASSENT-2 who received TNKase, 4,958 (59%) were under the age of 65; 2,256 (27%) were between the ages of 65 and 74; and 1,244 (15%) were 75 and over. The 30-day mortality rates by age were 2.5% in patients under the age of 65, 8.5% in patients between the ages of 65 and 74, and 16.2% in patients age 75 and over. The ICH rates were 0.4% in patients under the age of 65, 1.6% in patients between the ages of 65 and 74, and 1.7% in patients age 75 and over. The rates of any stroke were 1.0% in patients under the age of 65, 2.9% in patients between the ages of 65 and 74, and 3.0% in patients age 75 and over. Major bleeding rates, defined as bleeding requiring blood transfusion or leading to hemodynamic compromise, were 3.1% in patients under the age of 65, 6.4% in patients between the ages of 65 and 74, and 7.7% in patients age 75 and over. In elderly patients, the benefits of TNKase on mortality should be carefully weighed against the risk of increased adverse events, including bleeding.
-
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in Section PRECAUTIONS of the label:
- Hypersensitivity
Bleeding
The most frequent adverse reaction associated with TNKase is bleeding (see WARNINGS).
Should serious bleeding occur, concomitant heparin and antiplatelet therapy should be discontinued. Death or permanent disability can occur in patients who experience stroke or serious bleeding episodes.
For TNKase-treated patients in ASSENT-2, the incidence of intracranial hemorrhage was 0.9% and any stroke was 1.8%. The incidence of all strokes, including intracranial bleeding, increases with increasing age (see PRECAUTIONS: Geriatric Use).
In the ASSENT-2 study, the following bleeding events were reported (see Table 3).
Table 3 ASSENT-2 Non-ICH Bleeding Events TNKase
(n = 8461)Accelerated Activase
(n = 8488)Relative Risk for TNKase/Activase (95% CI) - * Major bleeding is defined as bleeding requiring blood transfusion or leading to hemodynamic compromise.
Major bleeding* 4.7% 5.9% 0.78 (0.69, 0.89) Minor bleeding 21.8% 23.0% 0.94 (0.89, 1.00) Units of transfused blood Any 4.3% 5.5% 0.77 (0.67, 0.89) 1–2 2.6% 3.2% >2 1.7% 2.2% Non-intracranial major bleeding and the need for blood transfusions were lower in patients treated with TNKase.
Types of major bleeding reported in 1% or more of the patients were hematoma (1.7%) and gastrointestinal tract (1%). Types of major bleeding reported in less than 1% of the patients were urinary tract, puncture site (including cardiac catheterization site), retroperitoneal, respiratory tract, and unspecified. Types of minor bleeding reported in 1% or more of the patients were hematoma (12.3%), urinary tract (3.7%), puncture site (including cardiac catheterization site) (3.6%), pharyngeal (3.1%), gastrointestinal tract (1.9%), epistaxis (1.5%), and unspecified (1.3%).
Other Adverse Reactions
The following adverse reactions have been reported among patients receiving TNKase in clinical trials. These reactions are frequent sequelae of the underlying disease, and the effect of TNKase on the incidence of these events is unknown.
These events include cardiogenic shock, arrhythmias, atrioventricular block, pulmonary edema, heart failure, cardiac arrest, recurrent myocardial ischemia, myocardial reinfarction, myocardial rupture, cardiac tamponade, pericarditis, pericardial effusion, mitral regurgitation, thrombosis, embolism, and electromechanical dissociation. These events can be life-threatening and may lead to death. Nausea and/or vomiting, hypotension, and fever have also been reported.
-
DOSAGE AND ADMINISTRATION
Dosage
TNKase® (Tenecteplase) is for intravenous administration only. The recommended total dose should not exceed 50 mg and is based upon patient weight.
A single bolus dose should be administered over 5 seconds based on patient weight. Treatment should be initiated as soon as possible after the onset of AMI symptoms (see CLINICAL STUDIES).
Dose Information Table Patient Weight
(kg)TNKase
(mg)Volume TNKase* to be administered (mL) - * From one vial of TNKase reconstituted with 10 mL SWFI.
<60 30 6 ≥60 to <70 35 7 ≥70 to <80 40 8 ≥80 to <90 45 9 ≥90 50 10 The safety and efficacy of TNKase have only been investigated with concomitant administration of heparin and aspirin as described in CLINICAL STUDIES.
THE
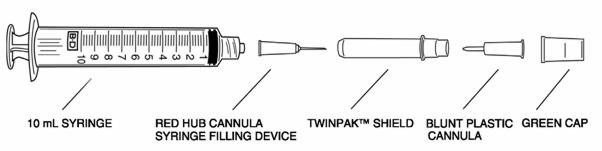
 ® 10 mL SYRINGE WITH TWINPAK™ DUAL CANNULA DEVICE
® 10 mL SYRINGE WITH TWINPAK™ DUAL CANNULA DEVICEReconstitution
NOTE: Read all instructions completely before beginning reconstitution and administration.
-
Remove the shield assembly from the supplied B-D® 10 mL syringe with TwinPak™ Dual Cannula Device (see figure) and aseptically withdraw 10 mL of Sterile Water for Injection (SWFI), USP, from the supplied diluent vial using the red hub cannula syringe filling device. Do not use Bacteriostatic Water for Injection, USP.
Note: Do not discard the shield assembly.
- Inject the entire contents of the syringe (10 mL) into the TNKase vial directing the diluent stream into the powder. Slight foaming upon reconstitution is not unusual; any large bubbles will dissipate if the product is allowed to stand undisturbed for several minutes.
- Gently swirl until contents are completely dissolved. DO NOT SHAKE. The reconstituted preparation results in a colorless to pale yellow transparent solution containing TNKase at 5 mg/mL at a pH of approximately 7.3. The osmolality of this solution is approximately 290 mOsm/kg.
- Determine the appropriate dose of TNKase (see Dose Information Table) and withdraw this volume (in milliliters) from the reconstituted vial with the syringe. Any unused solution should be discarded.
- Once the appropriate dose of TNKase is drawn into the syringe, stand the shield vertically on a flat surface (with green side down) and passively recap the red hub cannula.
- Remove the entire shield assembly, including the red hub cannula, by twisting counterclockwise. Note: The shield assembly also contains the clear-ended blunt plastic cannula; retain for split septum IV access.
Administration
- 1. The product should be visually inspected prior to administration for particulate matter and discoloration. TNKase may be administered as reconstituted at 5 mg/mL.
- 2. Precipitation may occur when TNKase is administered in an IV line containing dextrose. Dextrose-containing lines should be flushed with a saline-containing solution prior to and following single bolus administration of TNKase.
- 3. Reconstituted TNKase should be administered as a single IV bolus over 5 seconds.
- 4. Because TNKase contains no antibacterial preservatives, it should be reconstituted immediately before use. If the reconstituted TNKase is not used immediately, refrigerate the TNKase vial at 2–8°C (36–46°F) and use within 8 hours.
- 5. Although the supplied syringe is compatible with a conventional needle, this syringe is designed to be used with needleless IV systems. From the information below, follow the instructions applicable to the IV system in use.
Split septum IV system: - Remove the green cap.
- Attach the clear-ended blunt plastic cannula to the syringe.
- Remove the shield and use the blunt plastic cannula to access the split septum injection port.
- Because the blunt plastic cannula has two side ports, air or fluid expelled through the cannula will exit in two sideways directions; direct away from face or mucous membranes.
Luer-Lok® system: Connect syringe directly to IV port. Conventional needle (not supplied in this kit): Attach a large bore needle, e.g., 18 gauge, to the syringe's universal Luer‑Lok®. - 6. Dispose of the syringe, cannula and shield per established procedures.
-
HOW SUPPLIED
TNKase® (Tenecteplase) is supplied as a sterile, lyophilized powder in a 50 mg vial under partial vacuum. Each 50 mg vial of TNKase is packaged with one 10 mL vial of Sterile Water for Injection, USP for reconstitution, and the B-D® 10 mL syringe with TwinPak™ Dual Cannula Device. The following packaging configurations are available:
- With three alcohol prep pads (NDC: 50242-120-01).
- Without alcohol prep pads (NDC: 50242-120-47).
-
REFERENCES
- ASSENT-2 Investigators. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet1999;354:716-22.
- Cannon CP, Gibson CM, McCabe CH, Adgey AAJ, Schweiger MJ, Sequeira RF, et al. TNK-tissue plasminogen activator compared with front-loaded alteplase in acute myocardial infarction. Results of the TIMI 10B trial. Circulation1998;98:2805–14.
- Van de Werf F, Cannon CP, Luyten A, Houbracken K, McCabe CH, Berioli S, et al. Safety assessment of a single bolus administration of TNK tissue- plasminogen activator in acute myocardial infarction: the ASSENT-1 trial. Am Heart J1999;137:786–91.
-
SPL UNCLASSIFIED SECTION
TNKase® [Tenecteplase]
Manufactured by:
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-49904851700
Initial US Approval June 2000
Revision Date 08/2018
TNKase is a registered Trademark of Genentech, Inc.
©2018 Genentech, Inc.Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
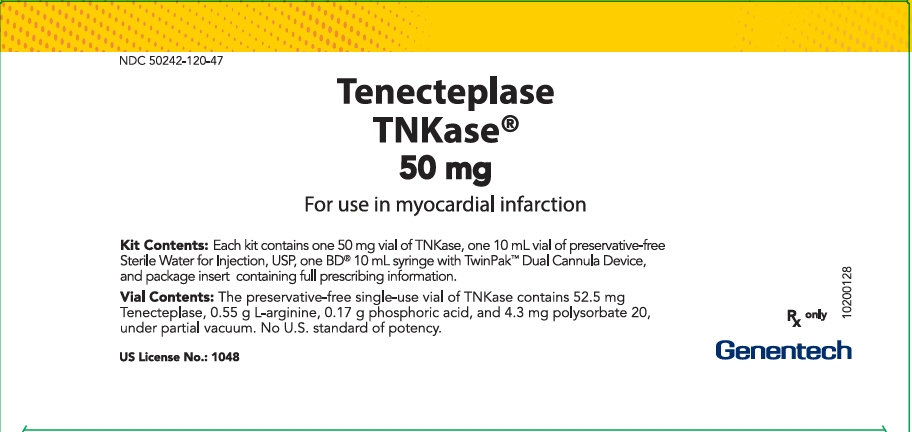
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 50242-120-47
Tenecteplase
TNKase®
50 mgFor use in myocardial infarction
Kit Contents: Each kit contains one 50 mg vial of TNKase, one 10 mL vial of preservative-free
Sterile Water for Injection, USP, one BD® 10 mL syringe with TwinPak™ Dual Cannula Device,
and package insert containing full prescribing information.Vial Contents: The preservative-free single-use vial of TNKase contains 52.5 mg
Tenecteplase, 0.55 g L-arginine, 0.17 g phosphoric acid, and 4.3 mg polysorbate 20,
under partial vacuum. No U.S. standard of potency.Rx only
US License No.: 1048
Genentech
10200128
-
INGREDIENTS AND APPEARANCE
TNKASE
tenecteplase kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50242-120 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-120-47 1 in 1 CARTON 11/07/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 50 mg Part 2 1 VIAL, SINGLE-USE 10 mL Part 1 of 2 TNKASE
tenecteplase injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 50242-037 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength tenecteplase (UNII: WGD229O42W) (tenecteplase - UNII:WGD229O42W) tenecteplase 52.5 mg in 50 mg Inactive Ingredients Ingredient Name Strength ARGININE (UNII: 94ZLA3W45F) 0.55 g in 50 mg PHOSPHORIC ACID (UNII: E4GA8884NN) 0.17 g in 50 mg POLYSORBATE 20 (UNII: 7T1F30V5YH) 4.3 mg in 50 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-037-06 1 in 1 CARTON 1 50 mg in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103909 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 50242-901 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-901-09 1 in 1 CARTON 1 10 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103909 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103909 11/07/2018 Labeler - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations Genentech, Inc. (SSF) 080129000 API MANUFACTURE(50242-120) , MANUFACTURE(50242-120) , ANALYSIS(50242-120) , PACK(50242-120) Establishment Name Address ID/FEI Business Operations Genentech, Inc. (Hillsboro) 833220176 LABEL(50242-120) , PACK(50242-120) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 323105205 ANALYSIS(50242-120) Establishment Name Address ID/FEI Business Operations Genentech, Inc. (OCN) 146373191 ANALYSIS(50242-120) Establishment Name Address ID/FEI Business Operations Roche Singapore Technical Operations Pte. Ltd. 937189173 ANALYSIS(50242-120)
Trademark Results [TNKase]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TNKASE 78423134 3048938 Live/Registered |
GENENTECH, INC. 2004-05-21 |
 TNKASE 75815961 not registered Dead/Abandoned |
GENENTECH, INC. 1999-10-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.