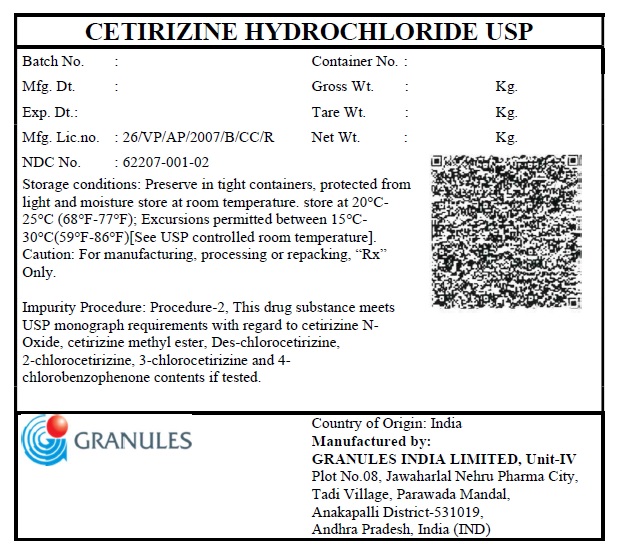
Cetirizine Hydrochloride, USP
Cetirizine Hydrochloride by
Drug Labeling and Warnings
Cetirizine Hydrochloride by is a Other medication manufactured, distributed, or labeled by Granules India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CETIRIZINE HYDROCHLORIDE- cetirizine hydrochloride powder
Granules India Limited
----------
Cetirizine Hydrochloride, USP
| CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride powder |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Granules India Limited (915000087) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India Limited | 650376275 | api manufacture(62207-001) , analysis(62207-001) , label(62207-001) , pack(62207-001) | |
Revised: 4/2024
Document Id: 1649a35f-e16a-8686-e063-6294a90a0a03
Set id: 73c32789-7930-4c59-99b5-b68a44186944
Version: 4
Effective Time: 20240417
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.