Triazolam by Rebel Distributors Corp TRIAZOLAM tablet
Triazolam by
Drug Labeling and Warnings
Triazolam by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Triazolam tablets contain triazolam, a triazolobenzodiazepine hypnotic agent.
Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.
The chemical name for triazolam is 8-chloro-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo-[4,3-α][1,4]benzodiazepine..
The structural formula is represented below:
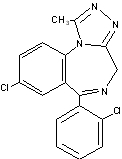
C17H12Cl2N4
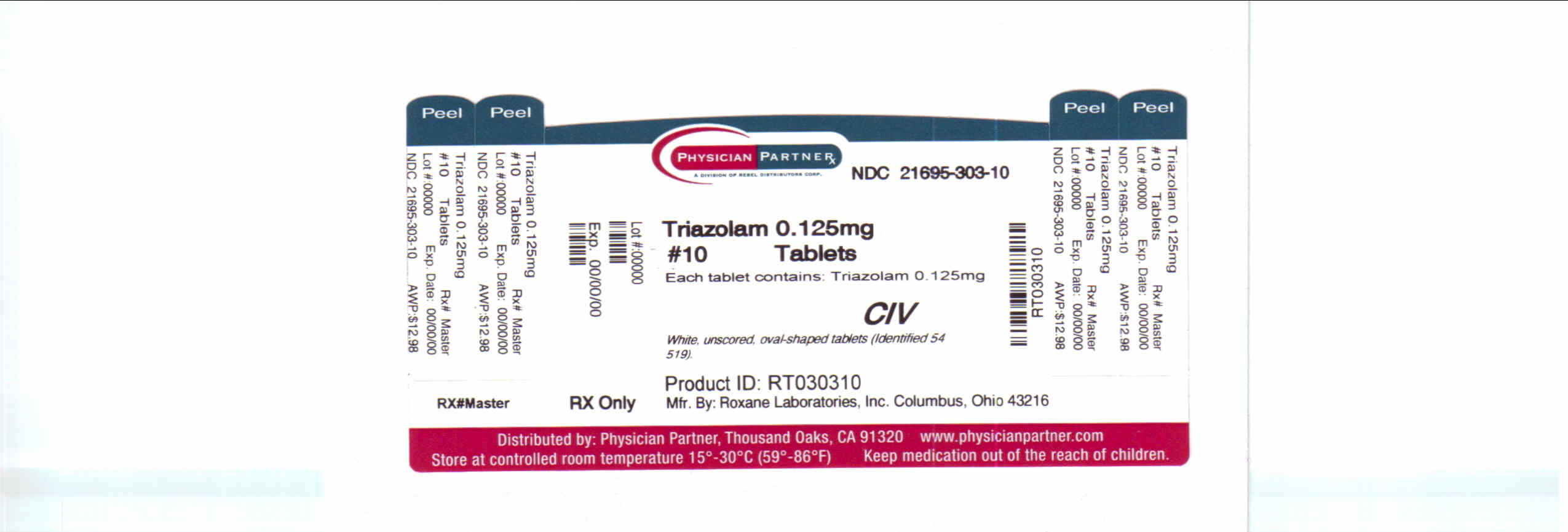
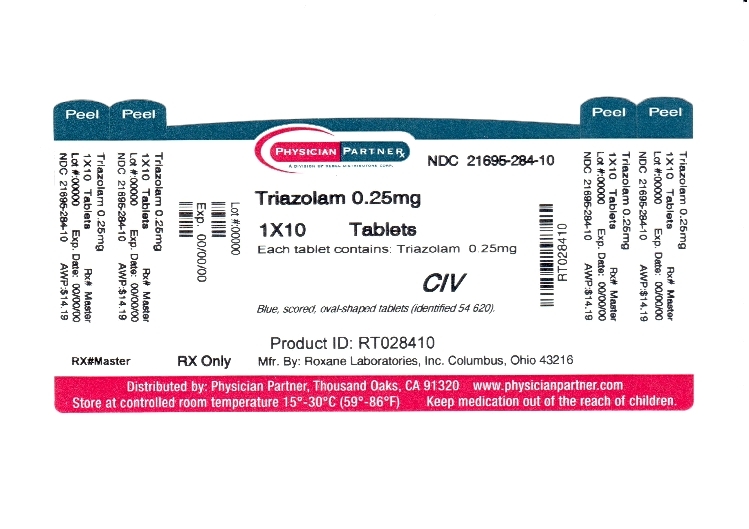
Each triazolam tablet, for oral administration, contains 0.125 mg or 0.25 mg of triazolam. Inactive ingredients: 0.125 mg-corn starch, docusate sodium, lactose (anhydrous), magnesium stearate, microcrystalline cellulose; 0.25 mg-corn starch, docusate sodium, FD&C Blue No. 1, lactose (anhydrous), magnesium stearate, microcrystalline cellulose.
-
CLINICAL PHARMACOLOGY
Triazolam is a hypnotic with a short mean plasma half-life reported to be in the range of 1.5 to 5.5 hours. In normal subjects treated for 7 days with four times the recommended dosage, there was no evidence of altered systemic bioavailability, rate of elimination, or accumulation. Peak plasma levels are reached within 2 hours following oral administration. Following recommended doses, triazolam peak plasma levels in the range of 1 to 6 ng/mL are seen. The plasma levels achieved are proportional to the dose given.
Triazolam and its metabolites, principally as conjugated glucuronides, which are presumably inactive, are excreted primarily in the urine. Only small amounts of unmetabolized triazolam appear in the urine. The two primary metabolites accounted for 79.9% of urinary excretion. Urinary excretion appeared to be biphasic in its time course.
Triazolam tablets 0.5 mg, in two separate studies, did not affect the prothrombin times or plasma warfarin levels in male volunteers administered sodium warfarin orally.
Extremely high concentrations of triazolam do not displace bilirubin bound to human serum albumin in vitro.
Triazolam 14C was administered orally to pregnant mice. Drug-related material appeared uniformly distributed in the fetus with 14C concentrations approximately the same as in the brain of the mother.
In sleep laboratory studies, triazolam tablets significantly decreased sleep latency, increased the duration of sleep, and decreased the number of nocturnal awakenings. After 2 weeks of consecutive nightly administration, the drug’s effect on total wake time is decreased, and the values recorded in the last third of the night approach baseline levels. On the first and/or second night after drug discontinuance (first or second post-drug night), total time asleep, percentage of time spent sleeping, and rapidity of falling asleep frequently were significantly less than on baseline (predrug) nights. This effect is often called “rebound” insomnia.
The type and duration of hypnotic effects and the profile of unwanted effects during administration of benzodiazepine drugs may be influenced by the biologic half-life of administered drug and any active metabolites formed. When half-lives are long, the drug or metabolites may accumulate during periods of nightly administration and be associated with impairments of cognitive and motor performance during waking hours; the possibility of interaction with other psychoactive drugs or alcohol will be enhanced. In contrast, if half-lives are short, the drug and metabolites will be cleared before the next dose is ingested, and carry-over effects related to excessive sedation or CNS depression should be minimal or absent. However, during nightly use for an extended period pharmacodynamic tolerance or adaptation to some effects of benzodiazepine hypnotics may develop. If the drug has a short half-life of elimination, it is possible that a relative deficiency of the drug or its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night’s use. This sequence of events may account for two clinical findings reported to occur after several weeks of nightly use of rapidly eliminated benzodiazepine hypnotics: 1) increased wakefulness during the last third of the night and 2) the appearance of increased signs of daytime anxiety after 10 days of continuous treatment.
In a study of elderly (62 to 83 years old) versus younger subjects (21 to 41 years old) who received triazolam at the same dose levels (0.125 mg and 0.25 mg), the elderly experienced both greater sedation and impairment of psychomotor performance. These effects resulted largely from higher plasma concentrations of triazolam in the elderly.
-
INDICATIONS AND USAGE
Triazolam Tablets are indicated for the short-term treatment of insomnia (generally 7 to 10 days). Use for more than 2 to 3 weeks requires complete reevaluation of the patient (see WARNINGS).
Prescriptions for triazolam should be written for short-term use (7 to 10 days) and it should not be prescribed in quantities exceeding a 1-month supply.
-
CONTRAINDICATIONS
Triazolam Tablets are contraindicated in patients with known hypersensitivity to this drug or other benzodiazepines.
Benzodiazepines may cause fetal damage when administered during pregnancy. An increased risk of congenital malformations associated with the use of diazepam and chlordiazepoxide during the first trimester of pregnancy has been suggested in several studies. Transplacental distribution has resulted in neonatal CNS depression following the ingestion of therapeutic doses of a benzodiazepine hypnotic during the last weeks of pregnancy.
Triazolam Tablets are contraindicated in pregnant women. If there is a likelihood of the patient becoming pregnant while receiving Triazolam Tablets, she should be warned of the potential risk to the fetus. Patients should be instructed to discontinue the drug prior to becoming pregnant. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered.
Triazolam Tablets are contraindicated with ketoconazole, itraconazole, and nefazodone, medications that significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP 3A) (see WARNINGS and PRECAUTIONS: Drug Interactions).
-
WARNINGS
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs. Because some of the important adverse effects of sedative-hypnotics appear to be dose related (see PRECAUTIONS and DOSAGE AND ADMINISTRATION), it is important to use the smallest possible effective dose, especially in the elderly.
Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a “sleep-driving” episode.
Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Severe Anaphylactic and Anaphylactoid Reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including triazolam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with triazolam should not be rechallenged with the drug.
Central Nervous System Manifestations
An increase in daytime anxiety has been reported for triazolam after as few as 10 days of continuous use. In some patients this may be a manifestation of interdose withdrawal (see CLINICAL PHARMACOLOGY). If increased daytime anxiety is observed during treatment, discontinuation of treatment may be advisable.
A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of benzodiazepine hypnotics including triazolam. Some of these changes may be characterized by decreased inhibition, e.g., aggressiveness and extroversion that seem excessive, similar to that seen with alcohol and other CNS depressants (e.g., sedative/hypnotics). Other kinds of behavioral changes have also been reported, for example, bizarre behavior, agitation, hallucinations, depersonalization. In primarily depressed patients, the worsening of depression, including suicidal thinking, has been reported in association with the use of benzodiazepines.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Because of its depressant CNS effects, patients receiving triazolam should be cautioned against engaging in hazardous occupations requiring complete mental alertness such as operating machinery or driving a motor vehicle. For the same reason, patients should be cautioned about the concomitant ingestion of alcohol and other CNS depressant drugs during treatment with triazolam tablets.
As with some, but not all benzodiazepines, anterograde amnesia of varying severity and paradoxical reactions have been reported following therapeutic doses of triazolam. Data from several sources suggest that anterograde amnesia may occur at a higher rate with triazolam than with other benzodiazepine hypnotics.
Triazolam Interaction With Drugs That Inhibit Metabolism Via Cytochrome P450 3A
The initial step in triazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP 3A). Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of triazolam. Consequently, triazolam should be avoided in patients receiving very potent inhibitors of CYP 3A. With drugs inhibiting CYP 3A to a lesser but still significant degree, triazolam should be used only with caution and consideration of appropriate dosage reduction. For some drugs, an interaction with triazolam has been quantified with clinical data; for other drugs, interactions are predicted from in vitro data and/or experience with similar drugs in the same pharmacologic class.
The following are examples of drugs known to inhibit the metabolism of triazolam and/or related benzodiazepines, presumably through inhibition of CYP 3A.
Potent CYP 3A Inhibitors
Potent inhibitors of CYP 3A that should not be used concomitantly with triazolam include ketoconazole, itraconazole, and nefazodone. Although data concerning the effects of azole-type antifungal agents other than ketoconazole and itraconazole on triazolam metabolism are not available, they should be considered potent CYP 3A inhibitors, and their co-administration with triazolam is not recommended (see CONTRAINDICATIONS).
Drugs Demonstrated to be CYP 3A Inhibitors on the Basis of Clinical Studies Involving Triazolam (caution and consideration of dose reduction are recommended during co-administration with triazolam)
Macrolide Antibiotics
Co-administration of erythromycin increased the maximum plasma concentration of triazolam by 46%, decreased clearance by 53%, and increased half-life by 35%; caution and consideration of appropriate triazolam dose reduction are recommended. Similar caution should be observed during co-administration with clarithromycin and other macrolide antibiotics.
Other Drugs Possibly Affecting Triazolam Metabolism
Other drugs possibly affecting triazolam metabolism by inhibition of CYP 3A are discussed in the PRECAUTIONS section (see PRECAUTIONS: Drug Interactions).
-
PRECAUTIONS
General
In elderly and/or debilitated patients it is recommended that treatment with triazolam tablets be initiated at 0.125 mg to decrease the possibility of development of oversedation, dizziness, or impaired coordination.
Some side effects reported in association with the use of triazolam appear to be dose related. These include drowsiness, dizziness. light-headedness, and amnesia.
The relationship between dose and what may be more serious behavioral phenomena is less certain. Specifically, some evidence, based on spontaneous marketing reports, suggests that confusion, bizarre or abnormal behavior, agitation, and hallucinations may also be dose related, but this evidence is inconclusive. In accordance with good medical practice it is recommended that therapy be initiated at the lowest effective dose (see DOSAGE AND ADMINISTRATION).
Cases of “traveler’s amnesia” have been reported by individuals who have taken triazolam to induce sleep while traveling, such as during an airplane flight. In some of these cases, insufficient time was allowed for the sleep period prior to awakening and before beginning activity. Also, the concomitant use of alcohol may have been a factor in some cases.
Caution should be exercised if triazolam is prescribed to patients with signs or symptoms of depression that could be intensified by hypnotic drugs. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in these patients, and the least amount of drug that is feasible should be available to the patient at any one time.
The usual precautions should be observed in patients with impaired renal or hepatic function, chronic pulmonary insufficiency, and sleep apnea. In patients with compromised respiratory function, respiratory depression and apnea have been reported infrequently.
Information for Patients
The Medication Guide for patients is included with this insert. To assure safe and effective use of triazolam, the information and instructions provided in this Medication Guide should be discussed with patients.
“Sleep-Driving” and Other Complex Behaviors
There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since “sleep-driving” can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative hypnotic. As with sleep-driving, patients usually do not remember these events.
Drug Interactions
Both pharmacodynamic and pharmacokinetic interactions have been reported with benzodiazepines. In particular, triazolam produces additive CNS depressant effects when co-administered with other psychotropic medications, anticonvulsants, antihistamines, ethanol, and other drugs which themselves produce CNS depression.
Drugs that Inhibit Triazolam Metabolism Via Cytochrome P450 3A
The initial step in triazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP 3A). Drugs which inhibit this metabolic pathway may have a profound effect on the clearance of triazolam (see CONTRAINDICATIONS and WARNINGS for additional drugs of this type). Triazolam is contraindicated with ketoconazole, itraconazole, and nefazodone.
Drugs and Other Substances Demonstrated to be CYP 3A Inhibitors of Possible Clinical Significance on the Basis of Clinical Studies Involving Triazolam (caution is recommended during co-administration with triazolam)
Isoniazid
Co-administration of isoniazid increased the maximum plasma concentration of triazolam by 20%, decreased clearance by 42%, and increased half-life by 31%.
Drugs Demonstrated to be CYP 3A Inhibitors on the Basis of Clinical Studies Involving Benzodiazepines Metabolized Similarly to Triazolam or on the Basis of In Vitro Studies with Triazolam or Other Benzodiazepines (caution is recommended during co-administration with triazolam)
Available data from clinical studies of benzodiazepines other than triazolam suggest a possible drug interaction with triazolam for the following: fluvoxamine, diltiazem, and verapamil. Data from in vitro studies of triazolam suggest a possible drug interaction with triazolam for the following: sertraline and paroxetine. Data from in vitro studies of benzodiazepines other than triazolam suggest a possible drug interaction with triazolam for the following: ergotamine, cyclosporine, amiodarone, nicardipine, and nifedipine. Caution is recommended during co-administration of any of these drugs with triazolam (see WARNINGS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential was observed in mice during a 24-month study with triazolam in doses up to 4,000 times the human dose.
Pregnancy
Non-Teratogenic Effects
It is to be considered that the child born of a mother who is on benzodiazepines may be at some risk for withdrawal symptoms from the drug, during the postnatal period. Also, neonatal flaccidity has been reported in an infant born of a mother who had been receiving benzodiazepines.
Nursing Mothers
Human studies have not been performed; however, studies in rats have indicated that triazolam and its metabolites are secreted in milk. Therefore, administration of triazolam to nursing mothers is not recommended.
Pediatric Use
Safety and effectiveness of triazolam in individuals below 18 years of age have not been established.
Geriatric Use
The elderly are especially susceptible to the dose related adverse effects of triazolam. They exhibit higher plasma triazolam concentrations due to reduced clearance of the drug as compared with younger subjects at the same dose. To minimize the possibility of development of oversedation, the smallest effective dose should be used (see CLINICAL PHARMACOLOGY, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION).
Tolerance/Withdrawal Phenomena
Some loss of effectiveness or adaptation to the sleep inducing effects of these medications may develop after nightly use for more than a few weeks and there may be a degree of dependence that develops. For the benzodiazepine sleeping pills that are eliminated quickly from the body, a relative deficiency of the drug may occur at some point in the interval between each night’s use. This can lead to (1) increased wakefulness during the last third of the night, and (2) the appearance of increased signs of daytime anxiety or nervousness. These two events have been reported in particular for triazolam.
There can be more severe ‘withdrawal’ effects when a benzodiazepine sleeping pill is stopped. Such effects can occur after discontinuing these drugs following use for only a week or two, but may be more common and more severe after longer periods of continuous use. One type of withdrawal phenomenon is the occurrence of what is known as ‘rebound insomnia’. That is, on the first few nights after the drug is stopped, insomnia is actually worse than before the sleeping pill was given. Other withdrawal phenomena following abrupt stopping of benzodiazepine sleeping pills range from mild unpleasant feelings to a major withdrawal syndrome which may include abdominal and muscle cramps, vomiting, sweating, tremor, and rarely, convulsions.
-
ADVERSE REACTIONS
During placebo-controlled clinical studies in which 1,003 patients received triazolam tablets, the most troublesome side effects were extensions of the pharmacologic activity of triazolam, e.g., drowsiness, dizziness, or light-headedness.
The figures cited below are estimates of untoward clinical event incidence among subjects who participated in the relatively short duration (i.e., 1 to 42 days) placebo-controlled clinical trials of triazolam. The figures cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice where patient characteristics and other factors often differ from those in clinical trials. These figures cannot be compared with those obtained from other clinical studies involving related drug products and placebo, as each group of drug trials is conducted under a different set of conditions.
Comparison of the cited figures, however, can provide the prescriber with some basis of estimating the relative contributions of drug and nondrug factors to the untoward event incidence rate in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient while inducing it in others. (For example, an anticholinergic, anxiolytic drug may relieve dry mouth [a sign of anxiety] in some subjects but induce it [an untoward event] in others.)
Triazolam Placebo Number of Patients 1003 997 % Patients Reporting: Central Nervous System Drowsiness 14 6.4 Headache 9.7 8.4 Dizziness 7.8 3.1 Nervousness 5.2 4.5 Light-headedness 4.9 0.9 Coordination disorders/ataxia 4.6 0.8 Gastrointestinal Nausea/vomiting 4.6 3.7 In addition to the relatively common (i.e., 1% or greater) untoward events enumerated above, the following adverse events have been reported less frequently (i.e., 0.9% to 0.5%): euphoria, tachycardia, tiredness, confusional states/memory impairment, cramps/pain, depression, visual disturbances.
Rare (i.e., less than 0.5%) adverse reactions included constipation, taste alterations, diarrhea, dry mouth, dermatitis/allergy, dreaming/nightmares, insomnia, paresthesia, tinnitus, dysesthesia, weakness, congestion, death from hepatic failure in a patient also receiving diuretic drugs.
In addition to these untoward events for which estimates of incidence are available, the following adverse events have been reported in association with the use of triazolam and other benzodiazepines: amnestic symptoms (anterograde amnesia with appropriate or inappropriate behavior), confusional states (disorientation, derealization, depersonalization, and/or clouding of consciousness), dystonia, anorexia, fatigue, sedation, slurred speech, jaundice, pruritus, dysarthria, changes in libido, menstrual irregularities, incontinence, and urinary retention. Other factors may contribute to some of these reactions, e.g., concomitant intake of alcohol or other drugs, sleep deprivation, an abnormal premorbid state, etc.
Other events reported include: paradoxical reactions such as stimulation, mania, an agitational state (restlessness, irritability, and excitation), increased muscle spasticity, sleep disturbances, hallucinations, delusions, aggressiveness, falling, somnambulism, syncope, inappropriate behavior and other adverse behavioral effects. Should these occur, use of the drug should be discontinued.
The following events have also been reported: chest pain, burning tongue/glossitis/stomatitis.
Laboratory analyses were performed on all patients participating in the clinical program for triazolam. The following incidences of abnormalities were observed in patients receiving triazolam and the corresponding placebo group. None of these changes were considered to be of physiological significance.
Triazolam Placebo Number of patients 380 361 % of Patients Reporting Low High Low High Hematology Hematocrit * * * * Hemoglobin * * * * Total WBC count 1.7 2.1 * 1.3 Neutrophil count 1.5 1.5 3.3 1 Lymphocyte count 2.3 4 3.1 3.8 Monocyte count 3.6 * 4.4 1.5 Eosinophil count 10.2 3.2 9.8 3.4 Basophil count 1.7 2.1 * 1.8 Urinalysis Albumin - 1.1 - * Sugar - * - * RBC/HPF - 2.9 - 2.9 WBC/HPF - 11.7 - 7.9 Blood chemistry Creatinine 2.4 1.9 3.6 1.5 Bilirubin * 1.5 1 * SGOT * 5.3 * 4.5 Alkaline phosphatase * 2.2 * 2.6 *Less than 1%
When treatment with triazolam is protracted, periodic blood counts, urinalysis, and blood chemistry analyses are advisable.
Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during therapy with triazolam and are of no known significance.
-
DRUG ABUSE AND DEPENDENCE
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
Controlled Substance
Triazolam is a controlled substance under the Controlled Substance Act, and triazolam tablets have been assigned to Schedule IV.
Abuse, Dependence, and Withdrawal
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting, sweating, dysphoria, perceptual disturbances and insomnia), have occurred following abrupt discontinuance of benzodiazepines, including triazolam. The more severe symptoms are usually associated with higher dosages and longer usage, although patients at therapeutic dosages given for as few as 1 to 2 weeks can also have withdrawal symptoms and in some patients there may be withdrawal symptoms (daytime anxiety, agitation) between nightly doses (see CLINICAL PHARMACOLOGY). Consequently, abrupt discontinuation should be avoided and a gradual dosage tapering schedule is recommended in any patient taking more than the lowest dose for more than a few weeks. The recommendation for tapering is particularly important in any patient with a history of seizure.
The risk of dependence is increased in patients with a history of alcoholism, drug abuse, or in patients with marked personality disorders. Such dependence-prone individuals should be under careful surveillance when receiving triazolam. As with all hypnotics, repeat prescriptions should be limited to those who are under medical supervision.
-
OVERDOSAGE
Because of the potency of triazolam, some manifestations of overdosage may occur at 2 mg, four times the maximum recommended therapeutic dose (0.5 mg).
Manifestations of overdosage with triazolam tablets include somnolence, confusion, impaired coordination, slurred speech, and ultimately, coma. Respiratory depression and apnea have been reported with overdosages of triazolam. Seizures have occasionally been reported after overdosages.
Death has been reported in association with overdoses of triazolam by itself, as it has with other benzodiazepines. In addition, fatalities have been reported in patients who have overdosed with a combination of a single benzodiazepine, including triazolam, and alcohol; benzodiazepine and alcohol levels seen in some of these cases have been lower than those usually associated with reports of fatality with either substance alone.
As in all cases of drug overdosage, respiration, pulse, and blood pressure should be monitored and supported by general measures when necessary. Immediate gastric lavage should be performed. An adequate airway should be maintained. Intravenous fluids may be administered.
Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
Experiments in animals have indicated that cardiopulmonary collapse can occur with massive intravenous doses of triazolam. This could be reversed with positive mechanical respiration and the intravenous infusion of norepinephrine bitartrate or metaraminol bitartrate. Hemodialysis and forced diuresis are probably of little value. As with the management of intentional overdosage with any drug, the physician should bear in mind that multiple agents may have been ingested by the patient.
The oral LD50 in mice is greater than 1,000 mg/kg and in rats is greater than 5,000 mg/kg.
-
DOSAGE AND ADMINISTRATION
It is important to individualize the dosage of triazolam tablets for maximum beneficial effect and to help avoid significant adverse effects.
The recommended dose for most adults is 0.25 mg before retiring. A dose of 0.125 mg may be found to be sufficient for some patients (e.g., low body weight). A dose of 0.5 mg should be used only for exceptional patients who do not respond adequately to a trial of a lower dose since the risk of several adverse reactions increases with the size of the dose administered. A dose of 0.5 mg should not be exceeded.
In geriatric and/or debilitated patients the recommended dosage range is 0.125 mg to 0.25 mg. Therapy should be initiated at 0.125 mg in these groups and the 0.25 mg dose should be used only for exceptional patients who do not respond to a trial of the lower dose. A dose of 0.25 mg should not be exceeded in these patients.
As with all medications, the lowest effective dose should be used.
-
HOW SUPPLIED
Triazolam Tablets, USP
0.125 mg white, unscored, oval-shaped tablets (Identified 54 519).
NDC: 21695-303-10: Bottles of 10 tablets.
NDC: 21695-303-30: Bottles of 30 tablets.
0.25 mg blue, scored, oval-shaped tablets (Identified 54 620).
NDC: 21695-284-10: Bottles of 10 tablets.
NDC: 21695-284-00: Bottles of 100 tablets.
-
MEDICATION GUIDE
Read this Medication Guide before you start taking triazolam and each time you get a refill.
There may be new information. This Medication Guide does not take the place of talking
to your doctor about your medical condition or treatment. You and your doctor should talk
about the SEDATIVE-HYPNOTIC when you start taking it and at regular checkups.
What is the most important information I should know about triazolam?
After taking a SEDATIVE-HYPNOTIC, you may get up out of bed while not beingfully awake and do an activity that you do not know you are doing. The next morning,you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with a SEDATIVE-HYPNOTIC. Reported activities include:
driving a car (“sleep-driving”)
making and eating food
talking on the phone
having sex
sleep-walking
Important:
1.Take triazolam exactly as prescribed
Do not take more triazolam than prescribed.
Take triazolam right before you get in bed, not sooner.
2.Do not take triazolam if you:
drink alcohol
take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take triazolam with your other medicines
cannot get a full night’s sleep
are pregnant or considering becoming pregnant
3.Call your doctor right away if you find out that you have done any of the aboveactivities after taking triazolam.
What are SEDATIVE-HYPNOTICS?
SEDATIVE-HYPNOTICs are sleep medicines. SEDATIVE-HYPNOTICs are used in adults for the treatment of the symptom of trouble falling asleep due to insomnia.
Triazolam is not indicated for use in children.
Elderly patients are especially susceptible to dose related adverse effects when taking triazolam.
Triazolam is a federally controlled substance (C-IV) because it can be abused or lead to dependence. Keep triazolam in a safe place to prevent misuse and abuse. Selling or giving away triazolam may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
Who should not take triazolam?
Do not take triazolam if you are allergic to anything in it. See the end of this Medication Guide for a complete list of ingredients in triazolam.
SEDATIVE-HYPNOTICS may not be right for you. Before starting SEDATIVE-HYPNOTICS,tell your doctor about all of your health conditions, including if you:
have a history of depression, mental illness, or suicidal thoughts
have a history of drug or alcohol abuse or addiction
have kidney or liver disease
have a lung disease or breathing problems
are pregnant, planning to become pregnant, or breastfeeding
Tell your doctor about all of the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Medicines can interact, sometimes causing side effects. Do not take SEDATIVE-HYPNOTICS with othermedicines that can make you sleepy.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
Triazolam should not be taken with potent inhibitors of CYP 3A including ketoconazole, itraconazole, nefazodone and possibly other azole-type antifungal agents.
How should I take triazolam?
Take triazolam exactly as prescribed. Do not take more triazolam than prescribed for you.
Take triazolam right before you get into bed. Or you can take triazolam after you have been in bed and have trouble falling asleep.
Do not take triazolam with or right after a meal.
Do not take triazolam unless you are able to get a full night’s sleep before youmust be active again.
Call your healthcare provider if your insomnia worsens or is not better within7 to 10 days. This may mean that there is another condition causing your sleep problem.
If you take too much triazolam or overdose, call your doctor or poison control center right away, or get emergency treatment.
What are the possible side effects of SEDATIVE-HYPNOTICS?
Serious side effects of SEDATIVE-HYPNOTICS include:
getting out of bed while not being fully awake and do an activity that you donot know you are doing. (See “What is the most important information I should know about SEDATIVE-HYPNOTICS?)
abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, and suicidal thoughts or actions.
memory loss, including “traveler’s amnesia”
anxiety
severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing, and nausea and vomiting. Get emergency medical help if you get these symptoms after taking SEDATIVE-HYPNOTICS.
Call your doctor right away if you have any of the above side effects or anyother side effects that worry you while using the SEDATIVE-HYPNOTIC.
Common side effects of triazolam include:
drowsiness
headache
dizziness
lightheadedness
“pins and needles” feelings on your skin
difficulty with coordination
You may still feel drowsy the next day after taking triazolam. Do not drive or doother dangerous activities (including operating machinery) after taking triazolamuntil you feel fully awake.
You may have withdrawal symptoms for 1 to 2 days when you stop taking the SEDATIVE-HYPNOTIC suddenly. Withdrawal symptoms include trouble sleeping, unpleasant feelings, stomach and muscle cramps, vomiting, sweating, shakiness, and seizures.
These are not all the side effects of SEDATIVE-HYPNOTICS. Ask your doctor or pharmacist for more information.
How should I store triazolam?
Store triazolam at room temperature between 59° and 86° F (15° to 30°C).
Protect from light.
Keep triazolam and all medicines out of the reach of children.
Do not use triazolam after the expiration date on the bottle.
General Information about SEDATIVE-HYPNOTICS
Medicines are sometimes prescribed for purposes not mentioned in a Medication Guide.
Do not use the SEDATIVE-HYPNOTIC for a condition for which it was not prescribed.
Do not give the SEDATIVE-HYPNOTIC to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about triazolam. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about triazolam that was written for healthcare professionals.
If you would like more information, contact 1-800-962-8364.
What are the ingredients in triazolam?
Active Ingredient: Triazolam
Inactive Ingredients: 0.125 mg-corn starch, docusate sodium, lactose (anhydrous),magnesium stearate, microcrystalline cellulose; 0.25 mg-corn starch,docusate sodium, FD&C Blue No. 1, lactose (anhydrous), magnesium stearate,microcrystalline cellulose.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
4076601//04 Revised February 2009
© RLI, 2009
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TRIAZOLAM
triazolam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-303(NDC:0054-4858) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAZOLAM (UNII: 1HM943223R) (TRIAZOLAM - UNII:1HM943223R) TRIAZOLAM 0.125 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DOCUSATE SODIUM (UNII: F05Q2T2JA0) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color WHITE Score no score Shape OVAL Size 8mm Flavor Imprint Code 54;519 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-303-10 10 in 1 BOTTLE 2 NDC: 21695-303-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074224 07/13/2009 TRIAZOLAM
triazolam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-284(NDC:0054-4859) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAZOLAM (UNII: 1HM943223R) (TRIAZOLAM - UNII:1HM943223R) TRIAZOLAM 0.25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DOCUSATE SODIUM (UNII: F05Q2T2JA0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color BLUE Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code 54;620 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-284-10 10 in 1 BOTTLE 2 NDC: 21695-284-00 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074224 06/01/1994 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.