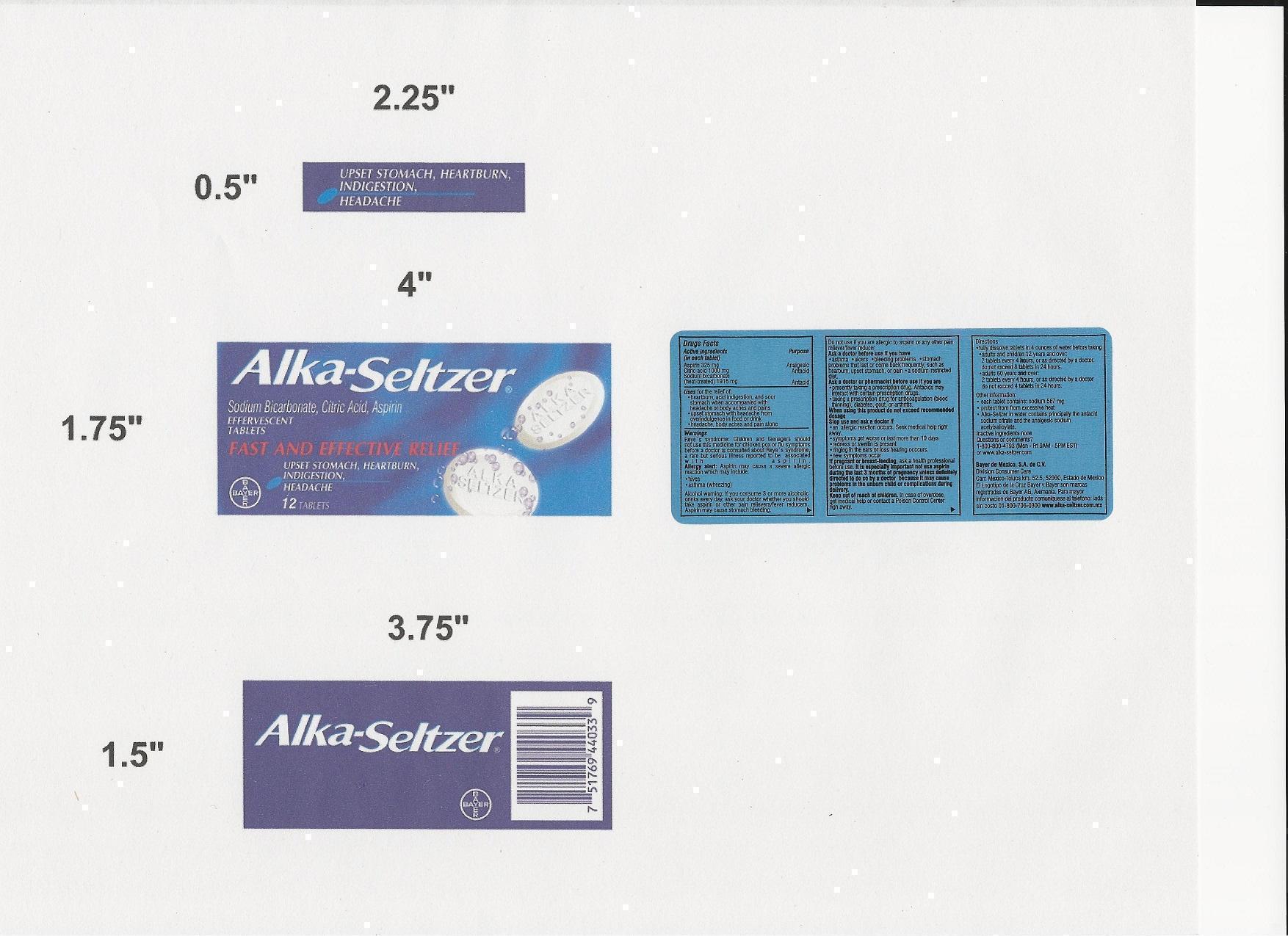
ALKA SELTZER- aspirin, anhydrous citric acid, sodium bicarbonate tablet, effervescent
Alka Seltzer by
Drug Labeling and Warnings
Alka Seltzer by is a Otc medication manufactured, distributed, or labeled by Salimex, S.A., Bayer de Mexico, S.A. de CV. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients (in each tablet)
- Purpose
- Keep out of reach of children
- Uses for the relief of:
-
Warnings
Reye's syndrome: Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye's syndrome, a rare but serious illness reported to be associated with aspirin.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- asthma (wheezing)
Alcohol warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
-
Directions
- fully dissolve 2 tablets in 4 ounces of water before taking
- adults and children 12 years and over: 2 tablets every 4 hours, or as directed by a doctor do not exceed 8 tablets in 24 hours
- adults 60 years and over 2 tablets every 4 hours, or as directed by a doctor do not exceed 4 tablets in 24 hours
- children under 12 years consult a doctor
Other information
- each tablet contains: sodium 567 mg
- store at room temperature. Avoid excessive heat.
- Alka-Seltzer in water contains principally the antacid sodium citrate and the analgesic sodium acetylsalicylate
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALKA SELTZER
aspirin, anhydrous citric acid, sodium bicarbonate tablet, effervescentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53666-403 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 1000 mg SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) BICARBONATE ION 1916 mg Product Characteristics Color white Score no score Shape ROUND Size 14mm Flavor Imprint Code ALKA;SELTZER Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53666-403-15 150 in 1 CASE 1 NDC: 53666-403-33 12 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 10/10/2012 ALKA SELTZER
aspirin, anhydrous citric acid, sodium bicarbonate tablet, effervescentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53666-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 1000 mg SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) BICARBONATE ION 1916 mg Product Characteristics Color white Score no score Shape ROUND Size 14mm Flavor Imprint Code ALKA;SELTZER Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53666-100-10 100 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 10/10/2012 Labeler - Salimex, S.A. (589201581) Establishment Name Address ID/FEI Business Operations Bayer de Mexico, S.A. de CV 588165709 manufacture(53666-403)
Trademark Results [Alka Seltzer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALKA SELTZER 78695208 not registered Dead/Abandoned |
Bayer HealthCare LLC 2005-08-18 |
 ALKA SELTZER 71677971 0613450 Dead/Expired |
MILES LABORATORIES, INC. 1954-12-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.