LYNPARZA- olaparib tablet, film coated
Lynparza by
Drug Labeling and Warnings
Lynparza by is a Prescription medication manufactured, distributed, or labeled by AstraZeneca Pharmaceuticals LP, AstraZeneca PLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LYNPARZA safely and effectively. See full prescribing information for LYNPARZA.
LYNPARZA® (olaparib) tablets, for oral use
Initial U.S. Approval: 2014RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Lynparza is a poly (ADP-ribose) polymerase (PARP) inhibitor indicated:
Ovarian cancer
- for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based chemotherapy. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza. (1.1, 2.1)
- for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer, who are in complete or partial response to platinum-based chemotherapy. (1.2)
- for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza. (1.3, 2.1)
Breast cancer
- for the treatment of adult patients with deleterious or suspected deleterious gBRCAm, HER2-negative metastatic breast cancer who have been treated with chemotherapy in the neoadjuvant, adjuvant or metastatic setting. Patients with hormone receptor (HR)-positive breast cancer should have been treated with a prior endocrine therapy or be considered inappropriate for endocrine therapy. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza. (1.4, 2.1)
Pancreatic cancer
- for the maintenance treatment of adult patients with deleterious or suspected deleterious gBRCAm metastatic pancreatic adenocarcinoma whose disease has not progressed on at least 16 weeks of a first-line platinum-based chemotherapy regimen. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza. (1.5, 2.1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 150 mg, 100 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML): Occurred in <1.5% of patients exposed to Lynparza monotherapy and the majority of events had a fatal outcome. Monitor patients for hematological toxicity at baseline and monthly thereafter. Discontinue if MDS/AML is confirmed. (5.1)
- Pneumonitis: Occurred in <1% of patients exposed to Lynparza, and some cases were fatal. Interrupt treatment if pneumonitis is suspected. Discontinue if pneumonitis is confirmed. (5.2)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of the potential risk to a fetus and to use effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (≥10%) in clinical trials were nausea, fatigue (including asthenia), vomiting, abdominal pain, anemia, diarrhea, dizziness, neutropenia, leukopenia, nasopharyngitis/upper respiratory tract infection/influenza, respiratory tract infection, arthralgia/myalgia, dysgeusia, headache, dyspepsia, decreased appetite, constipation, stomatitis, dyspnea and thrombocytopenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Lactation: Advise women not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
1.2 Maintenance Treatment of Recurrent Ovarian Cancer
1.3 Advanced Germline BRCA-mutated Ovarian Cancer After 3 or More Lines of Chemotherapy
1.4 Germline BRCA-mutated HER2-negative Metastatic Breast Cancer
1.5 First-Line Maintenance Treatment of Germline BRCA-mutated Metastatic Pancreatic Adenocarcinoma
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Dosage Modifications for Adverse Reactions
2.4 Dosage Modifications for Concomitant Use with Strong or Moderate CYP3A Inhibitors
2.5 Dosage Modifications for Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
5.2 Pneumonitis
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Use with Anticancer Agents
7.2 Effect of Other Drugs on Lynparza
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
14.2 Maintenance Treatment of Recurrent Ovarian Cancer
14.3 Advanced Germline BRCA-mutated Ovarian Cancer Treated with 3 or More Prior Lines of Chemotherapy
14.4 Treatment of Germline BRCA-mutated HER2-negative Metastatic Breast Cancer
14.5 First-Line Maintenance Treatment of Germline BRCA-mutated Metastatic Pancreatic Adenocarcinoma
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
Lynparza is indicated for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based chemotherapy. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza [see Dosage and Administration (2.1)].
1.2 Maintenance Treatment of Recurrent Ovarian Cancer
Lynparza is indicated for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer, who are in complete or partial response to platinum-based chemotherapy.
1.3 Advanced Germline BRCA-mutated Ovarian Cancer After 3 or More Lines of Chemotherapy
Lynparza is indicated for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza [see Dosage and Administration (2.1)].
1.4 Germline BRCA-mutated HER2-negative Metastatic Breast Cancer
Lynparza is indicated for the treatment of adult patients with deleterious or suspected deleterious gBRCAm, HER2-negative metastatic breast cancer, who have been treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting. Patients with hormone receptor (HR)-positive breast cancer should have been treated with a prior endocrine therapy or be considered inappropriate for endocrine therapy. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza [see Dosage and Administration (2.1)].
1.5 First-Line Maintenance Treatment of Germline BRCA-mutated Metastatic Pancreatic Adenocarcinoma
Lynparza is indicated for the maintenance treatment of adult patients with deleterious or suspected deleterious gBRCAm metastatic pancreatic adenocarcinoma whose disease has not progressed on at least 16 weeks of a first-line platinum-based chemotherapy regimen. Select patients for therapy based on an FDA-approved companion diagnostic for Lynparza [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
Select patients with advanced ovarian cancer who are in complete or partial response to first-line platinum-based chemotherapy for maintenance treatment with Lynparza based on the presence of deleterious or suspected deleterious germline or somatic BRCA mutation [see Clinical Studies (14.1)]. Information on FDA-approved tests for the detection of BRCA mutations is available at http://www.fda.gov/companiondiagnostics.
Germline BRCAm Advanced Ovarian Cancer, HER2-negative Metastatic Breast Cancer, and Metastatic Pancreatic Adenocarcinoma
Select patients for treatment with Lynparza based on the presence of deleterious or suspected deleterious germline BRCA mutation [see Clinical Studies(14.3, 14.4,14.5)]. Information on FDA-approved tests for the detection of BRCA mutations is available at http://www.fda.gov/companiondiagnostics.
2.2 Recommended Dosage
The recommended dosage of Lynparza is 300 mg taken orally twice daily, with or without food.
First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
Continue treatment until disease progression, unacceptable toxicity, or completion of 2 years of treatment. Patients with a complete response (no radiological evidence of disease) at 2 years should stop treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating healthcare provider can derive further benefit from continuous treatment, can be treated beyond 2 years.
Recurrent Ovarian Cancer, Germline BRCAm Advanced Ovarian Cancer, HER2-negative Metastatic Breast Cancer, and Metastatic Pancreatic Adenocarcinoma
Continue treatment until disease progression or unacceptable toxicity for:
- Maintenance treatment of recurrent ovarian cancer
- Advanced germline BRCA-mutated ovarian cancer
- Germline BRCA-mutated HER-2 negative metastatic breast cancer
- First-line maintenance treatment of germline BRCA-mutated metastatic pancreatic adenocarcinoma.
If a patient misses a dose of Lynparza, instruct patient to take their next dose at its scheduled time.
Instruct patients to swallow tablets whole. Do not chew, crush, dissolve, or divide tablet.
2.3 Dosage Modifications for Adverse Reactions
To manage adverse reactions, consider interruption of treatment or dose reduction. The recommended dose reduction is 250 mg taken twice daily.
If a further dose reduction is required, then reduce to 200 mg taken twice daily.
2.4 Dosage Modifications for Concomitant Use with Strong or Moderate CYP3A Inhibitors
Avoid concomitant use of strong or moderate CYP3A inhibitors with Lynparza.
If concomitant use cannot be avoided, reduce Lynparza dosage to:
- 100 mg twice daily when used concomitantly with a strong CYP3A inhibitor.
- 150 mg twice daily when used concomitantly with a moderate CYP3A inhibitor.
After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume the Lynparza dose taken prior to initiating the CYP3A inhibitor [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
2.5 Dosage Modifications for Renal Impairment
Moderate Renal Impairment
In patients with moderate renal impairment (CLcr 31-50 mL/min), reduce the Lynparza dosage to 200 mg orally twice daily [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
Overall, the incidence of Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML) in patients treated with Lynparza monotherapy in clinical trials, including long-term follow up, was <1.5% (30/2527) and the majority of events had a fatal outcome. Of these, 22/26 patients had a documented BRCA mutation, 2 patients had gBRCA wildtype and in 2 patients the BRCA mutation status was unknown. Additional cases of MDS/AML have been documented in patients treated with Lynparza in combination studies and in postmarketing reports. The duration of therapy with Lynparza in patients who developed secondary MDS/cancer-therapy related AML varied from <6 months to >2 years. All of these patients had received previous chemotherapy with platinum agents and/or other DNA damaging agents including radiotherapy. Some of these patients also had a history of more than one primary malignancy or of bone marrow dysplasia.
Do not start Lynparza until patients have recovered from hematological toxicity caused by previous chemotherapy (≤Grade 1). Monitor complete blood count for cytopenia at baseline and monthly thereafter for clinically significant changes during treatment. For prolonged hematological toxicities, interrupt Lynparza and monitor blood counts weekly until recovery. If the levels have not recovered to Grade 1 or less after 4 weeks, refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics. If MDS/AML is confirmed, discontinue Lynparza.
5.2 Pneumonitis
Pneumonitis, including fatal cases, occurred in <1% of patients treated with Lynparza. If patients present with new or worsening respiratory symptoms such as dyspnea, cough and fever, or a radiological abnormality occurs, interrupt Lynparza treatment and promptly assess the source of the symptoms. If pneumonitis is confirmed, discontinue Lynparza treatment and treat the patient appropriately.
5.3 Embryo-Fetal Toxicity
Lynparza can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals. In an animal reproduction study, administration of olaparib to pregnant rats during the period of organogenesis caused teratogenicity and embryo-fetal toxicity at exposures below those in patients receiving the recommended human dose of 300 mg twice daily. Apprise pregnant women of the potential hazard to a fetus and the potential risk for loss of the pregnancy. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of Lynparza. Based on findings from genetic toxicity and animal reproduction studies, advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for 3 months following the last dose of Lynparza [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling:
- Myelodysplastic Syndrome/Acute Myeloid Leukemia [see Warnings and Precautions (5.1)]
- Pneumonitis [see Warnings and Precautions (5.2)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
SOLO-1
The safety of Lynparza for the maintenance treatment of patients with BRCA-mutated advanced ovarian cancer following first-line treatment with platinum-based chemotherapy was investigated in SOLO-1 [see Clinical Studies (14.1)]. Patients received Lynparza tablets 300 mg orally twice daily (n=260) or placebo (n=130) until disease progression or unacceptable toxicity. The median duration of study treatment was 25 months for patients who received Lynparza and 14 months for patients who received placebo.
Among patients who received Lynparza, dose interruptions due to an adverse reaction of any grade occurred in 52% and dose reductions due to an adverse reaction occurred in 28%. The most frequent adverse reactions leading to dose interruption or reduction of Lynparza were anemia (23%), nausea (14%), and vomiting (10%). Discontinuation due to adverse reactions occurred in 12% of patients receiving Lynparza. The most frequent adverse reactions that led to discontinuation of Lynparza were fatigue (3.1%), anemia (2.3%), and nausea (2.3%).
Tables 1 and 2 summarize adverse reactions and laboratory abnormalities in SOLO-1.
Table 1 Adverse Reactions* in SOLO-1 (≥10% of Patients Who Received Lynparza) Adverse Reaction Lynparza tablets
n=260Placebo
n=130All Grades
(%)Grades
3 – 4 (%)All
Grades (%)Grades
3 – 4 (%)- * Graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.0.
- † Includes abdominal pain, abdominal pain lower, abdominal pain upper, abdominal distension, abdominal discomfort, abdominal tenderness
- ‡ Includes colitis, diarrhea, gastroenteritis
- § Includes stomatitis, aphthous ulcer; mouth ulceration
- ¶ Includes: asthenia, fatigue, lethargy, malaise
- # Includes neutropenia, febrile neutropenia
- Þ Includes leukopenia, white blood cell count decreased
- ß Includes platelet count decreased, thrombocytopenia
- à Includes: urosepsis, urinary tract infection, urinary tract pain, pyuria
- è Includes dyspnea and dyspnea exertional
Gastrointestinal Disorders
Nausea
77
1
38
0
Abdominal pain†
45
2
35
1
Vomiting
40
0
15
1
Diarrhea‡
37
3
26
0
Constipation
28
0
19
0
Dyspepsia
17
0
12
0
Stomatitis§
11
0
2
0
General Disorders and Administration Site Conditions
Fatigue¶
67
4
42
2
Blood and Lymphatic System Disorders
Anemia
38
21
9
2
Neutropenia#
17
6
7
3
LeukopeniaÞ
13
3
8
0
Thrombocytopeniaß
11
1
4
2
Infections and Infestations
Upper respiratory tract infection/ influenza/nasopharyngitis/bronchitis
28
0
23
0
UTIà
13
1
7
0
Nervous System Disorders
Dysgeusia
26
0
4
0
Dizziness
20
0
15
1
Metabolism and Nutrition Disorders
Decreased appetite
20
0
10
0
Respiratory, Thoracic and Mediastinal Disorders
Dyspneaè
15
0
6
0
In addition, the adverse reactions observed in SOLO-1 that occurred in <10% of patients receiving Lynparza were increased blood creatinine (8%), lymphopenia (6%), hypersensitivity (2%), dermatitis (1%), and increased mean cell volume (0.4%).
Table 2 Laboratory Abnormalities Reported in ≥25% of Patients in SOLO-1 - * Patients were allowed to enter clinical studies with laboratory values of CTCAE Grade 1.
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
Laboratory Parameter*
Lynparza tablets
n†=260
Placebo
n†=130
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Decrease in hemoglobin
87
19
63
2
Increase in mean corpuscular volume
87
-
43
-
Decrease in leukocytes
70
7
52
1
Decrease in lymphocytes
67
14
29
5
Decrease in absolute neutrophil count
51
9
38
6
Decrease in platelets
35
1
20
2
Increase in serum creatinine
34
0
18
0
Maintenance Treatment of Recurrent Ovarian Cancer
SOLO-2
The safety of Lynparza for the maintenance treatment of patients with platinum sensitive gBRCAm ovarian cancer was investigated in SOLO-2 [see Clinical Studies (14.2)]. Patients received Lynparza tablets 300 mg orally twice daily (n=195) or placebo (n=99) until disease progression or unacceptable toxicity. The median duration of study treatment was 19.4 months for patients who received Lynparza and 5.6 months for patients who received placebo. Among patients who received Lynparza, dose interruptions due to an adverse reaction of any grade occurred in 45% and dose reductions due to an adverse reaction occurred in 27%. The most frequent adverse reactions leading to dose interruption or reduction of Lynparza were anemia (22%), neutropenia (9%), and fatigue/asthenia (8%). Discontinuation due to an adverse reaction occurred in 11% of patients receiving Lynparza.
Tables 3 and 4 summarize adverse reactions and laboratory abnormalities in SOLO-2.
Table 3 Adverse Reactions* in SOLO-2 (≥20% of Patients Who Received Lynparza) - * Graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.0.
- † Represents grouped term consisting of abscess oral, aphthous ulcer, gingival abscess, gingival disorder, gingival pain, gingivitis, mouth ulceration, mucosal infection, mucosal inflammation, oral candidiasis, oral discomfort, oral herpes, oral infection, oral mucosal erythema, oral pain, oropharyngeal discomfort, and oropharyngeal pain.
- ‡ Represents grouped term consisting of anemia, hematocrit decreased, hemoglobin decreased, iron deficiency, mean cell volume increased and red blood cell count decreased.
Adverse Reaction
Lynparza tablets
n=195
Placebo
n=99
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Gastrointestinal Disorders
Nausea
76
3
33
0
Vomiting
37
3
19
1
Diarrhea
33
2
22
0
Stomatitis†
20
1
16
0
General Disorders and Administration Site Conditions
Fatigue including asthenia
66
4
39
2
Blood and Lymphatic Disorders
Anemia‡
44
20
9
2
Infections and Infestations
Nasopharyngitis/URI/sinusitis/ rhinitis/influenza
36
0
29
0
Musculoskeletal and Connective Tissue Disorders
Arthralgia/myalgia
30
0
28
0
Nervous System Disorders
Dysgeusia
27
0
7
0
Headache
26
1
14
0
Metabolism and Nutrition Disorders
Decreased appetite
22
0
11
0
In addition, the adverse reactions observed in SOLO-2 that occurred in <20% of patients receiving Lynparza were neutropenia (19%), cough (18%), leukopenia (16%), hypomagnesemia (14%), thrombocytopenia (14%), dizziness (13%), dyspepsia (11%), increased creatinine (11%), edema (8%), rash (6%), and lymphopenia (1%).
Table 4 Laboratory Abnormalities Reported in ≥25% of Patients in SOLO-2 - * Patients were allowed to enter clinical studies with laboratory values of CTCAE Grade 1.
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
- ‡ Represents the proportion of subjects whose mean corpuscular volume was > upper limit of normal (ULN).
Laboratory Parameter*
Lynparza tablets
n†=195
Placebo
n†=99
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Increase in mean corpuscular volume‡
89
-
52
-
Decrease in hemoglobin
83
17
69
0
Decrease in leukocytes
69
5
48
1
Decrease in lymphocytes
67
11
37
1
Decrease in absolute neutrophil count
51
7
34
3
Increase in serum creatinine
44
0
29
0
Decrease in platelets
42
2
22
1
Study 19
The safety of Lynparza as maintenance monotherapy was evaluated in patients with platinum sensitive ovarian cancer who had received 2 or more previous platinum containing regimens in Study 19 [see Clinical Studies (14.2)]. Patients received Lynparza capsules 400 mg orally twice daily (n=136) or placebo (n=128). At the time of final analysis, the median duration of exposure was 8.7 months in patients who received Lynparza and 4.6 months in patients who received placebo.
Adverse reactions led to dose interruptions in 35% of patients receiving Lynparza; dose reductions in 26% and discontinuation in 6% of patients receiving Lynparza.
Tables 5 and 6 summarize adverse reactions and laboratory abnormalities in Study 19.
Table 5 Adverse Reactions* in Study 19 (≥20% of Patients Who Received Lynparza) - * Graded according to NCI CTCAE v4.0.
- † Represents grouped terms of related terms that reflect the medical concept of the adverse reaction.
Adverse Reaction
Lynparza capsules
n=136
Placebo
n=128
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Gastrointestinal Disorders
Nausea
71
2
36
0
Vomiting
35
2
14
1
Diarrhea
28
2
25
2
Constipation
22
1
12
0
Dyspepsia
20
0
9
0
General Disorders and Administration Site Conditions
Fatigue (including asthenia)
63
9
46
3
Blood and Lymphatic Disorders
Anemia†
23
7
7
1
Infections and Infestations
Respiratory tract infection
22
2
11
0
Metabolism and Nutrition Disorders
Decreased appetite
21
0
13
0
Nervous System Disorders
Headache
21
0
13
1
In addition, the adverse reactions in Study 19 that occurred in <20% of patients receiving Lynparza were dysgeusia (16%), dizziness (15%), dyspnea (13%), pyrexia (10%), stomatitis (9%), edema (9%), increase in creatinine (7%), neutropenia (5%), thrombocytopenia (4%), leukopenia (2%), and lymphopenia (1%).
Table 6 Laboratory Abnormalities Reported in ≥25% of Patients in Study 19 - * Patients were allowed to enter clinical studies with laboratory values of CTCAE Grade 1.
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
- ‡ Represents the proportion of subjects whose mean corpuscular volume was > ULN.
Laboratory Parameter*
Lynparza capsules
n†=136
Placebo
n†=129
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Decrease in hemoglobin
82
8
58
1
Increase in mean corpuscular volume‡
82
-
51
-
Decrease in leukocytes
58
4
37
2
Decrease in lymphocytes
52
10
32
3
Decrease in absolute neutrophil count
47
7
40
2
Increase in serum creatinine
45
0
14
0
Decrease in platelets
36
4
18
0
Advanced Germline BRCA-mutated Ovarian Cancer After 3 or More Lines of Chemotherapy
Pooled Data
The safety of Lynparza was investigated in 223 patients (pooled from 6 studies) with gBRCAm advanced ovarian cancer who had received 3 or more prior lines of chemotherapy [see Clinical Studies (14.3)]. Patients received Lynparza capsules 400 mg orally twice daily until disease progression or unacceptable tolerability. The median exposure to Lynparza in these patients was 5.2 months.
There were 8 (4%) patients with adverse reactions leading to death, two were attributed to acute leukemia, and one each was attributed to COPD, cerebrovascular accident, intestinal perforation, pulmonary embolism, sepsis, and suture rupture. Adverse reactions led to dose interruption in 40% of patients, dose reduction in 4%, and discontinuation in 7%.
Tables 7 and 8 summarize the adverse reactions and laboratory abnormalities from the pooled studies.
Table 7 Adverse Reactions Reported in Pooled Data (≥20% of Patients Who Received Lynparza) Adverse Reaction
Lynparza capsules
n=223
Grades 1-4
(%)
Grades 3-4
(%)
General Disorders
Fatigue/asthenia
66
8
Gastrointestinal Disorders
Nausea
64
3
Vomiting
43
4
Diarrhea
31
1
Dyspepsia
25
0
Decreased appetite
22
1
Blood and Lymphatic Disorders
Anemia
34
18
Infections and Infestations
Nasopharyngitis/URI
26
0
Musculoskeletal and Connective Tissue Disorders
Arthralgia/musculoskeletal pain
21
0
Myalgia
22
0
Table 8 Laboratory Abnormalities Reported in ≥25% of Patients in Pooled Data - * Patients were allowed to enter clinical studies with laboratory values of CTCAE Grade 1.
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
Laboratory Parameter*
Lynparza capsules
n†=223
Grades 1-4
(%)
Grades 3-4
(%)
Decrease in hemoglobin
90
15
Mean corpuscular volume elevation
57
-
Decrease in lymphocytes
56
17
Decrease in platelets
30
3
Increase in creatinine
30
2
Decrease in absolute neutrophil count
25
7
The following adverse reactions and laboratory abnormalities have been identified in ≥10 to <20% of the 223 patients receiving Lynparza and not included in the table: cough (16%), constipation (16%), dysgeusia (16%), headache (15%), peripheral edema (14%), back pain (14%), urinary tract infection (14%), dyspnea (13%) and dizziness (11%).
The following adverse reactions and laboratory abnormalities have been identified in <10% of the 223 patients receiving Lynparza and not included in the table: leukopenia (9%), pyrexia (8%), peripheral neuropathy (5%), hypomagnesemia (5%), rash (5%), stomatitis (4%) and venous thrombosis (including pulmonary embolism) (1%).
Germline BRCA-mutated HER2-negative Metastatic Breast Cancer
OlympiAD
The safety of Lynparza was evaluated in gBRCAm patients with HER2-negative metastatic breast cancer who had previously received up to two lines of chemotherapy for the treatment of metastatic disease in OlympiAD [see Clinical Studies (14.4)]. Patients received either Lynparza tablets 300 mg orally twice daily (n=205) or a chemotherapy (capecitabine, eribulin, or vinorelbine) of the healthcare provider’s choice (n=91) until disease progression or unacceptable toxicity. The median duration of study treatment was 8.2 months in patients who received Lynparza and 3.4 months in patients who received chemotherapy.
Among patients who received Lynparza, dose interruptions due to an adverse reaction of any grade occurred in 35% and dose reductions due to an adverse reaction occurred in 25%. Discontinuation due to an adverse reaction occurred in 5% of patients receiving Lynparza.
Tables 9 and 10 summarize the adverse reactions and laboratory abnormalities in OlympiAD.
Table 9 Adverse Reactions* in OlympiAD (≥20% of Patients Who Received Lynparza) - * Graded according to NCI CTCAE v4.0.
- † Represents grouped terms consisting of anemia (anemia erythropenia, hematocrit decreased, hemoglobin decreased and red blood cell count decreased).
- ‡ Represents grouped terms consisting of neutropenia (febrile neutropenia, granulocyte count decreased, granulocytopenia, neutropenia, neutropenic infection, neutropenic sepsis, neutrophil count decreased).
- § Represents grouped terms consisting of leukopenia (leukopenia and white blood cell count decreased).
- ¶ Represents grouped terms consisting of bronchitis, influenza, lower respiratory tract infection, nasopharyngitis, pharyngitis, respiratory tract infection, rhinitis, sinusitis, upper respiratory tract infection, and upper respiratory tract infection bacterial.
Adverse Reaction
Lynparza tablets
n=205
Chemotherapy
n=91
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Gastrointestinal Disorders
Nausea
58
0
35
1
Vomiting
30
0
15
1
Diarrhea
21
1
22
0
Blood and Lymphatic Disorders
Anemia†
40
16
26
4
Neutropenia‡
27
9
50
26
Leukopenia§
25
5
31
13
General Disorders and Administration Site Conditions
Fatigue (including asthenia)
37
4
36
1
Infections and Infestations
Respiratory tract infection¶
27
1
22
0
Nervous System Disorders
Headache
20
1
15
2
In addition, adverse reactions in OlympiAD that occurred in <20% of patients receiving Lynparza were cough (18%), decreased appetite (16%), thrombocytopenia (11%), dysgeusia (9%), lymphopenia (8%), dyspepsia (8%), dizziness (7%), stomatitis (7%), upper abdominal pain (7%), rash (5%), increase in serum creatinine (3%), and dermatitis (1%).
Table 10 Laboratory Abnormalities Reported in ≥25% of Patients in OlympiAD - * Patients were allowed to enter clinical studies with laboratory values of CTCAE Grade 1.
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
- ‡ Represents the proportion of subjects whose mean corpuscular volume was > ULN.
Laboratory Parameter*
Lynparza tablets
n† = 205
Chemotherapy
n† = 91
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Decrease in hemoglobin
82
17
66
3
Decrease in lymphocytes
73
21
63
3
Decrease in leukocytes
71
8
70
23
Increase in mean corpuscular volume‡
71
-
33
-
Decrease in absolute neutrophil count
46
11
65
38
Decrease in platelets
33
3
28
0
First-line Maintenance Treatment of Germline BRCA-mutated Metastatic Pancreatic Adenocarcinoma
POLO
The safety of Lynparza as maintenance treatment of germline BRCA-mutated metastatic pancreatic adenocarcinoma following first-line treatment with platinum-based chemotherapy was evaluated in POLO [see Clinical Studies (14.5)]. Patients received Lynparza tablets 300 mg orally twice daily (n=90) or placebo (n=61) until disease progression or unacceptable toxicity. Among patients receiving Lynparza, 34% were exposed for 6 months or longer and 25% were exposed for greater than one year.
Among patients who received Lynparza, dosage interruptions due to an adverse reaction of any grade occurred in 35% and dosage reductions due to an adverse reaction occurred in 17%. The most frequent adverse reactions leading to dosage interruption or reduction in patients who received Lynparza were anemia (11%), vomiting (5%), abdominal pain (4%), asthenia (3%), and fatigue (2%). Discontinuation due to adverse reactions occurred in 6% of patients receiving Lynparza. The most frequent adverse reaction that led to discontinuation of Lynparza was fatigue (2.2%).
Tables 11 and 12 summarize the adverse reactions and laboratory abnormalities in patients in POLO.
Table 11 Adverse Reactions*in POLO (Occurring in ≥10% of Patients who Received Lynparza) Adverse Reaction Lynparza tablets
(n=91)†Placebo
(n=60)†All Grades
(%)Grades
3 – 4 (%)All
Grades (%)Grades
3 – 4 (%)- * Graded according to NCI CTCAE, version 4.0
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
- ‡ Includes asthenia and fatigue
- § Includes abdominal pain, abdominal pain upper, abdominal pain lower
- ¶ Includes stomatitis and mouth ulceration
- # Includes neutropenia, febrile neutropenia and neutrophil count decreased
- Þ Includes platelets count decreased and thrombocytopenia
- ß Includes rash erythematous, rash macular and rash maculo-papular
- à Includes dyspnea and dyspnea exertional
General Disorders and Administration Site Conditions
Fatigue‡
60
5
35
2
Gastrointestinal Disorders
Nausea
45
0
23
2
Abdominal pain§
34
2
37
5
Diarrhea
29
0
15
0
Constipation
23
0
10
0
Vomiting
20
1
15
2
Stomatitis¶
10
0
5
0
Blood and Lymphatic System Disorders
Anemia
27
11
17
3
Thrombocytopenia#
14
3
7
0
NeutropeniaÞ
12
4
8
3
Metabolism and Nutrition Disorders
Decreased appetite
25
3
7
0
Musculoskeletal and Connective Tissue Disorders
Back pain
19
0
17
2
Arthralgia
15
1
10
0
Skin and Subcutaneous Tissue Disorder
Rashß
15
0
5
0
Respiratory, Thoracic and Mediastinal Disorders
Dyspneaà
13
0
5
2
Infections and Infestations
Nasopharyngitis
12
0
3
0
Nervous System Disorders
Dysgeusia
11
0
5
0
In addition, the adverse reactions observed in POLO that occurred in <10% of patients receiving Lynparza were cough (9%), abdominal pain upper (7%), blood creatinine increased (7%), dizziness (7%), headache (7%), dyspepsia (5%), leukopenia (5%), hypersensitivity (2%) and lymphopenia (2%).
Table 12 Laboratory Abnormalities Reported in ≥25% of Patients in POLO - * Patients were allowed to enter POLO with hemoglobin ≥9 g/dL (CTCAE Grade 2) and other laboratory values of CTCAE Grade 1.
- † This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
- ‡ Represents the proportion of subjects whose mean corpuscular volume was > ULN.
Laboratory Parameter*
Lynparza tablets
n†=91
Placebo
n†=60
Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
Increase in serum creatinine
99
2
85
0
Decrease in hemoglobin
86
11
65
0
Increase in mean corpuscular volume‡
71
-
30
-
Decrease in lymphocytes
61
9
27
0
Decrease in platelets
56
2
39
0
Decrease in leukocytes
50
3
23
0
Decrease in absolute neutrophil count
25
3
10
0
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Lynparza. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity (rash/dermatitis).
-
7 DRUG INTERACTIONS
7.1 Use with Anticancer Agents
Clinical studies of Lynparza with other myelosuppressive anticancer agents, including DNA damaging agents, indicate a potentiation and prolongation of myelosuppressive toxicity.
7.2 Effect of Other Drugs on Lynparza
Strong and Moderate CYP3A Inhibitors
Coadministration of CYP3A inhibitors can increase olaparib concentrations, which may increase the risk for adverse reactions [see Clinical Pharmacology (12.3)]. Avoid coadministration of strong or moderate CYP3A inhibitors. If the strong or moderate inhibitor must be coadministered, reduce the dose of Lynparza [see Dosage and Administration (2.4)].
Strong and Moderate CYP3A Inducers
Concomitant use with a strong or moderate CYP3A inducer decreased olaparib exposure, which may reduce Lynparza efficacy [see Clinical Pharmacology (12.3)]. Avoid coadministration of strong or moderate CYP3A inducers.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals and its mechanism of action [see Clinical Pharmacology (12.1)], Lynparza can cause fetal harm when administered to a pregnant woman. There are no available data on Lynparza use in pregnant women to inform the drug-associated risk. In an animal reproduction study, the administration of olaparib to pregnant rats during the period of organogenesis caused teratogenicity and embryo-fetal toxicity at exposures below those in patients receiving the recommended human dose of 300 mg twice daily (see Data). Apprise pregnant women of the potential hazard to the fetus and the potential risk for loss of the pregnancy.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. The estimated background risk in the U.S. general population of major birth defects is 2-4%; and the risk for spontaneous abortion is approximately 15-20% in clinically recognized pregnancies.
Data
Animal Data
In a fertility and early embryonic development study in female rats, olaparib was administered orally for 14 days before mating through to Day 6 of pregnancy, which resulted in increased post-implantation loss at a dose level of 15 mg/kg/day (with maternal systemic exposures approximately 7% of the human exposure (AUC0-24h) at the recommended dose).
In an embryo-fetal development study, pregnant rats received oral doses of 0.05 and 0.5 mg/kg/day olaparib during the period of organogenesis. A dose of 0.5 mg/kg/day (with maternal systemic exposures approximately 0.18% of human exposure (AUC0-24h) at the recommended dose) caused embryo-fetal toxicities including increased post-implantation loss and major malformations of the eyes (anophthalmia, microphthalmia), vertebrae/ribs (extra rib or ossification center; fused or absent neural arches, ribs, and sternebrae), skull (fused exoccipital) and diaphragm (hernia). Additional abnormalities or variants included incomplete or absent ossification (vertebrae/sternebrae, ribs, limbs) and other findings in the vertebrae/sternebrae, pelvic girdle, lung, thymus, liver, ureter and umbilical artery. Some findings noted above in the eyes, ribs and ureter were observed at a dose of 0.05 mg/kg/day olaparib at lower incidence.
8.2 Lactation
Risk Summary
No data are available regarding the presence of olaparib in human milk, or on its effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in the breastfed infants from Lynparza, advise a lactating woman not to breastfeed during treatment with Lynparza and for one month after receiving the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Recommend pregnancy testing for females of reproductive potential prior to initiating treatment with Lynparza.
Contraception
Females
Lynparza can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with Lynparza and for at least 6 months following the last dose.
Males
Based on findings in genetic toxicity and animal reproduction studies, advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for 3 months following the last dose of Lynparza. Advise male patients not to donate sperm during therapy and for 3 months following the last dose of Lynparza [see Use in Specific Populations (8.1) and Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of Lynparza have not been established in pediatric patients.
8.5 Geriatric Use
Of the 687 patients with advanced solid tumors who received Lynparza tablets 300 mg orally twice daily as monotherapy, 146 (21%) patients were aged ≥65 years, and this included 29 (4%) patients who were aged ≥75 years. No patients were aged ≥85 years. No overall differences in the safety or effectiveness of Lynparza were observed between these patients and younger patients.
8.6 Renal Impairment
No dosage modification is recommended in patients with mild renal impairment (CLcr 51 to 80 mL/min estimated by Cockcroft-Gault). Reduce Lynparza dosage to 200 mg twice daily in patients with moderate renal impairment (CLcr 31 to 50 mL/min) [see Dosage and Administration (2.5)]. There are no data in patients with severe renal impairment or end-stage disease (CLcr ≤30 mL/min) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No adjustment to the starting dose is required in patients with mild or moderate hepatic impairment (Child-Pugh classification A and B). There are no data in patients with severe hepatic impairment (Child-Pugh classification C) [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
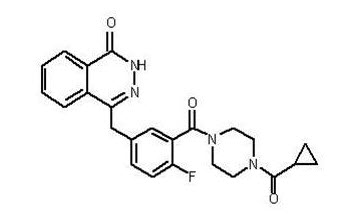
Olaparib is a poly (ADP-ribose) polymerase (PARP) inhibitor. The chemical name is 4-[(3-{[4-(cyclopropylcarbonyl)piperazin-1-yl]carbonyl}-4-fluorophenyl)methyl]phthalazin-1(2H)-one. The empirical molecular formula for Lynparza is C24H23FN4O3 and the relative molecular mass is 434.46. It has the following chemical structure:
Olaparib is a crystalline solid, is non-chiral and shows pH-independent low solubility across the physiological pH range.
Lynparza (olaparib) tablets for oral use contain 100 mg or 150 mg of olaparib. Inactive ingredients in the tablet core are copovidone, mannitol, colloidal silicon dioxide and sodium stearyl fumarate. The tablet coating consists of hypromellose, polyethylene glycol 400, titanium dioxide, ferric oxide yellow and ferrosoferric oxide (150 mg tablet only).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Olaparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1, PARP2, and PARP3. PARP enzymes are involved in normal cellular functions, such as DNA transcription and DNA repair. Olaparib has been shown to inhibit growth of select tumor cell lines in vitro and decrease tumor growth in mouse xenograft models of human cancer, both as monotherapy or following platinum-based chemotherapy. Increased cytotoxicity and anti-tumor activity following treatment with olaparib were noted in cell lines and mouse tumor models with deficiencies in BRCA and non-BRCA proteins involved in the homologous recombination repair (HRR) of DNA damage and correlated with platinum response. In vitro studies have shown that olaparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes, resulting in DNA damage and cancer cell death.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of olaparib on cardiac repolarization was assessed in 119 patients following a single dose of 300 mg and in 109 patients following multiple dosing of 300 mg twice daily. No clinically relevant effect of olaparib on QT interval was observed.
12.3 Pharmacokinetics
The area under the curve (AUC) of olaparib increases approximately proportionally following administration of single doses of 25 mg to 450 mg (0.08 to 1.5 times the recommended dose) and maximal concentrations (Cmax) increased slightly less than proportionally for the same dose range. Olaparib showed time-dependent pharmacokinetics and an AUC mean accumulation ratio of 1.8 is observed at steady state following a dose of 300 mg twice daily.
The mean (CV%) olaparib Cmax is 5.8 μg/mL (36%) and AUC is 42 μg*h/mL (51%) following a single 300 mg dose. The mean steady state olaparib Cmax and AUC is 7.7 μg/mL (40%) and 49 μg*h/mL (52%), following a dose of 300 mg twice daily.
Absorption
Following oral administration of olaparib, the median time to peak plasma concentration is 1.5 hours.
Effect of Food
Co-administration of a high fat and high calorie meal (800-1000 kcal, 50% of the calorie content made up from fat) with olaparib slowed the rate (tmax delayed by 2.5 hours) of absorption, but did not significantly alter the extent of olaparib absorption (mean AUC increased by approximately 8%).
Distribution
The mean (± standard deviation) apparent volume of distribution of olaparib is 158 ± 136 L following a single 300 mg dose of Lynparza. The protein binding of olaparib is approximately 82% in vitro.
Elimination
The mean (± standard deviation) terminal plasma half-life of olaparib is 14.9 ± 8.2 hours and the apparent plasma clearance is 7.4 ± 3.9 L/h following a single 300 mg dose of Lynparza.
Metabolism
Olaparib is metabolized by cytochrome P450 (CYP) 3A in vitro.
Following an oral dose of radiolabeled olaparib to female patients, unchanged olaparib accounted for 70% of the circulating radioactivity in plasma. It was extensively metabolized with unchanged drug accounting for 15% and 6% of radioactivity in urine and feces, respectively. The majority of the metabolism is attributable to oxidation reactions with a number of the components produced undergoing subsequent glucuronide or sulfate conjugation.
Excretion
Following a single dose of radiolabeled olaparib, 86% of the dosed radioactivity was recovered within a 7-day collection period, 44% via the urine and 42% via the feces. The majority of the material was excreted as metabolites.
Specific Populations
Patients with Renal Impairment
In a renal impairment trial, the mean AUC increased by 24% and Cmax by 15%, when olaparib was dosed in patients with mild renal impairment (CLcr=51-80 mL/min defined by the Cockcroft-Gault equation; n=13) and by 44% and 26%, respectively, when olaparib was dosed in patients with moderate renal impairment (CLcr=31-50 mL/min; n=13), compared to those with normal renal function (CLcr ≥81 mL/min; n=12). There was no evidence of a relationship between the extent of plasma protein binding of olaparib and creatinine clearance. There are no data in patients with severe renal impairment or end-stage renal disease (CLcr ≤30 mL/min).
Patients with Hepatic Impairment
In a hepatic impairment trial, the mean AUC increased by 15% and the mean Cmax increased by 13% when olaparib was dosed in patients with mild hepatic impairment (Child-Pugh classification A; n=10) and the mean AUC increased by 8% and the mean Cmax decreased by 13% when olaparib was dosed in patients with moderate hepatic impairment (Child-Pugh classification B; n=8), compared to patients with normal hepatic function (n=13). Hepatic impairment had no effect on the protein binding of olaparib and, therefore, total plasma exposure was representative of free drug. There are no data in patients with severe hepatic impairment (Child-Pugh classification C).
Drug Interaction Studies
Clinical Studies
CYP3A Inhibitors: Concomitant use of itraconazole (strong CYP3A inhibitor) increased olaparib Cmax by 42% and AUC by 170%. Concomitant use of fluconazole (moderate CYP3A inhibitor) is predicted to increase olaparib Cmax by 14% and AUC by 121%.
CYP3A Inducers: Concomitant use of rifampicin (strong CYP3A inducer) decreased olaparib Cmax by 71% and AUC by 87%. Concomitant use of efavirenz (moderate CYP3A inducer) is predicted to decrease olaparib Cmax by 31% and AUC by 60%.
In vitro Studies
CYP Enzymes: Olaparib is both an inhibitor and inducer of CYP3A and an inducer of CYP2B6. Olaparib is predicted to be a weak CYP3A inhibitor in humans.
UGT Enzymes: Olaparib is an inhibitor of UGT1A1.
Transporters: Olaparib is an inhibitor of BCRP, OATP1B1, OCT1, OCT2, OAT3, MATE1, and MATE2K. Olaparib is a substrate and inhibitor of the efflux transporter P-gp. The potential for olaparib to induce P-gp has not been evaluated.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with olaparib.
Olaparib was clastogenic in an in vitro chromosomal aberration assay in mammalian Chinese hamster ovary (CHO) cells and in an in vivo rat bone marrow micronucleus assay. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of olaparib and indicates potential for genotoxicity in humans. Olaparib was not mutagenic in a bacterial reverse mutation (Ames) test.
In a fertility study, female rats received oral olaparib at doses of 0.05, 0.5, and 15 mg/kg/day for at least 14 days before mating through the first week of pregnancy. There were no adverse effects on mating and fertility rates at doses up to 15 mg/kg/day (maternal systemic exposures approximately 7% of the human exposure (AUC0-24h) at the recommended dose).
In a male fertility study, olaparib had no effect on mating and fertility in rats at oral doses up to 40 mg/kg/day following at least 70 days of olaparib treatment (with systemic exposures of approximately 5% of the human exposure (AUC0-24h) at the recommended dose).
-
14 CLINICAL STUDIES
14.1 First-Line Maintenance Treatment of BRCA-mutated Advanced Ovarian Cancer
The efficacy of Lynparza was evaluated in SOLO-1 (NCT01844986), a randomized (2:1), double-blind, placebo-controlled, multi-center trial in patients with BRCA-mutated advanced ovarian, fallopian tube, or primary peritoneal cancer following first-line platinum-based chemotherapy. Patients were randomized to receive Lynparza tablets 300 mg orally twice daily or placebo. Treatment was continued for up to 2 years or until disease progression or unacceptable toxicity; however, patients with evidence of disease at 2 years, who in the opinion of the treating healthcare provider could derive further benefit from continuous treatment, could be treated beyond 2 years. Randomization was stratified by response to first-line platinum-based chemotherapy (complete or partial response). The major efficacy outcome was investigator-assessed progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
A total of 391 patients were randomized, 260 to Lynparza and 131 to placebo. The median age of patients treated with Lynparza was 53 years (range: 29 to 82) and 53 years (range: 31 to 84) among patients on placebo. The ECOG performance status (PS) was 0 in 77% of patients receiving Lynparza and 80% of patients receiving placebo. Of all patients, 82% were White, 36% were enrolled in the U.S. or Canada, and 82% were in complete response to their most recent platinum-based regimen. The majority of patients (n=389) had germline BRCA mutation (gBRCAm), and 2 patients had somatic BRCAm (sBRCAm).
Of the 391 patients randomized in SOLO-1, 386 were retrospectively or prospectively tested with a Myriad BRACAnalysis test and 383 patients were confirmed to have deleterious or suspected deleterious gBRCAm status; 253 were randomized to the Lynparza arm and 130 to the placebo arm. Two out of 391 patients randomized in SOLO-1 were confirmed to have sBRCAm based on an investigational Foundation Medicine tissue test.
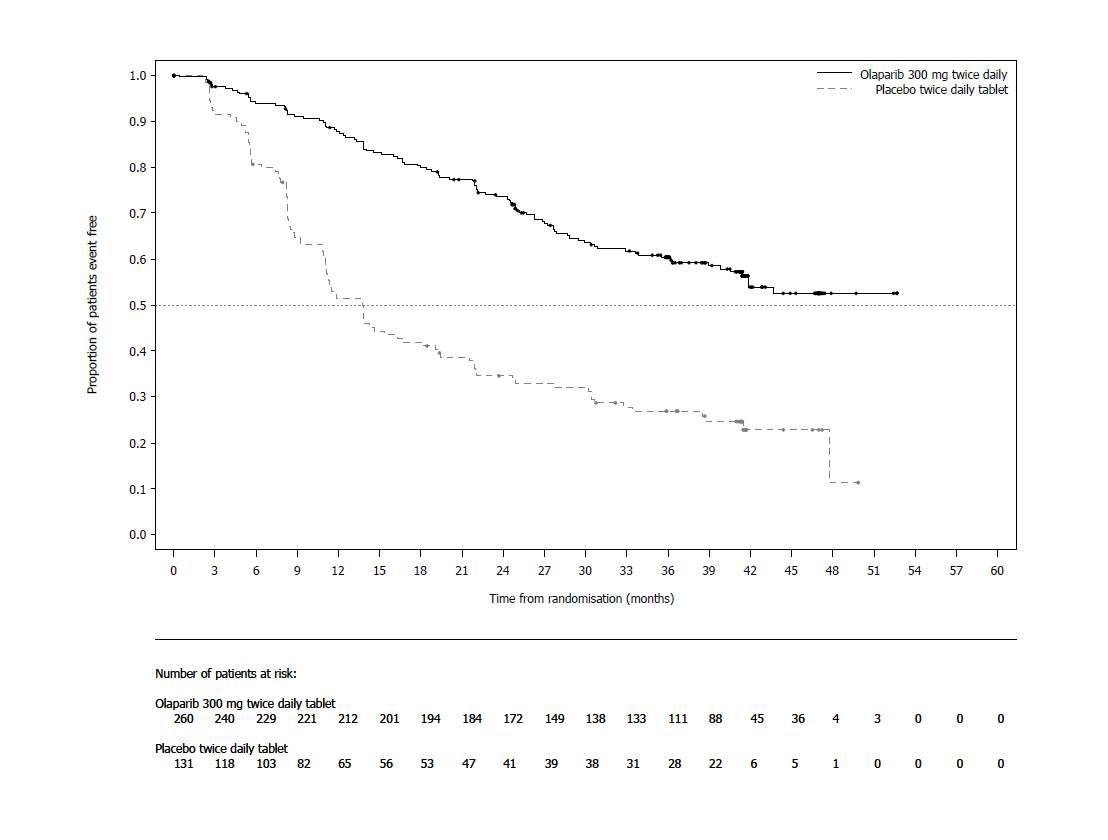
SOLO-1 demonstrated a statistically significant improvement in investigator-assessed PFS for Lynparza compared to placebo. Results from a blinded independent review were consistent. At the time of the analysis of PFS, overall survival (OS) data were not mature (21% of patients had died). Efficacy results are presented in Table 13 and Figure 1.
Table 13 Efficacy Results - SOLO-1 (Investigator Assessment) - * Median follow up of 41 months in both treatment arms.
- † A value <1 favors olaparib. Hazard ratio from a Cox proportional hazards model including response to previous platinum chemotherapy (complete response versus partial response) as a covariate.
- ‡ The p-value is derived from a stratified log-rank test.
Lynparza tablets
(n=260)
Placebo
(n=131)
Progression-Free Survival*
Number of events (%)
102 (39%)
96 (73%)
Median, months
NR
13.8
Hazard ratio† (95% CI)
0.30 (0.23, 0.41)
p-value‡
<0.0001
NR not reached; CI Confidence Interval.
Figure 1 Kaplan-Meier Curves of Investigator-Assessed Progression-Free Survival-SOLO-1
14.2 Maintenance Treatment of Recurrent Ovarian Cancer
The efficacy of Lynparza was investigated in two randomized, placebo-controlled, double-blind, multi-center studies in patients with recurrent ovarian cancers who were in response to platinum-based therapy.
SOLO-2
The efficacy of Lynparza was evaluated in SOLO-2 (NCT01874353), a randomized (2:1) double-blind, placebo-controlled trial in patients with gBRCAm ovarian, fallopian tube, or primary peritoneal cancer. Patients were randomized to Lynparza tablets 300 mg orally twice daily or placebo until unacceptable toxicity or progressive disease. Randomization was stratified by response to last platinum chemotherapy (complete versus partial) and time to disease progression in the penultimate platinum-based chemotherapy prior to enrollment (6-12 months versus >12 months). All patients had received at least two prior platinum-containing regimens and were in response (complete or partial) to their most recent platinum-based regimen. The major efficacy outcome measure was investigator-assessed PFS evaluated according to RECIST, version 1.1. An additional efficacy outcome measure was OS.
A total of 295 patients were randomized, 196 to Lynparza and 99 to placebo. The median age of patients treated with Lynparza was 56 years (range: 28 to 83) and 56 years (range: 39 to 78) among patients treated with placebo. The ECOG PS was 0 in 83% of patients receiving Lynparza and 78% of patients receiving placebo. Of all patients, 89% were White, 17% were enrolled in the U.S. or Canada, 47% were in complete response to their most recent platinum-based regimen, and 40% had a progression-free interval of 6-12 months since their penultimate platinum regimen. Prior bevacizumab therapy was reported for 17% of those treated with Lynparza and 20% of those receiving placebo. Approximately 44% of patients on the Lynparza arm and 37% on placebo had received three or more lines of platinum-based treatment.
All patients had a deleterious or suspected deleterious germline BRCA mutation as detected either by a local test (n=236) or central Myriad CLIA test (n=59), subsequently confirmed by BRACAnalysis CDx® (n=286).
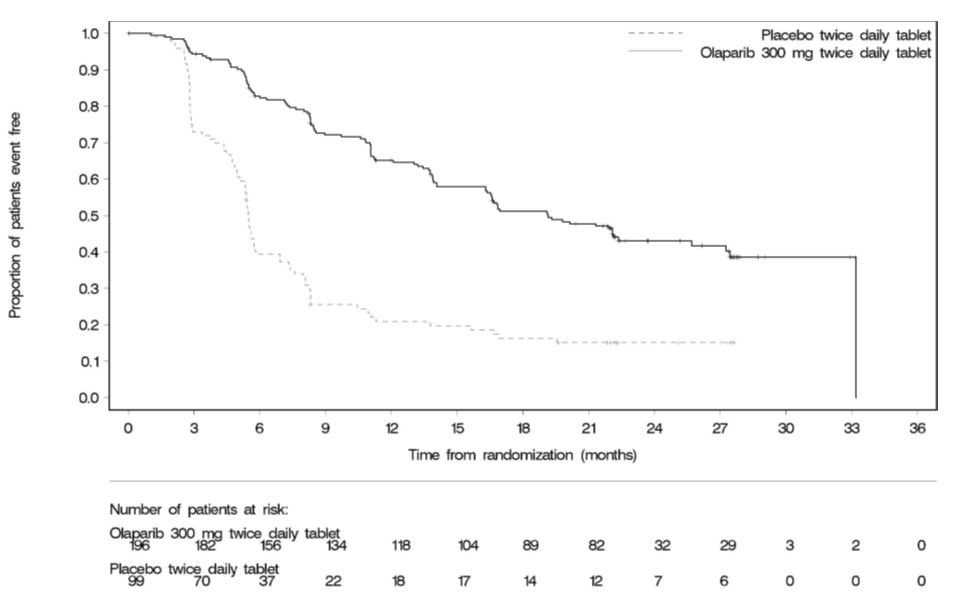
SOLO-2 demonstrated a statistically significant improvement in investigator-assessed PFS in patients randomized to Lynparza as compared with placebo. Results from a blinded independent review were consistent. At the time of the analysis of PFS, OS data were not mature with 24% of events. Efficacy results are presented in Table 14 and Figure 2.
Table 14 Efficacy Results - SOLO-2 (Investigator Assessment) - * Hazard ratio from a Cox proportional hazards model including response to last platinum chemotherapy (complete response versus partial response) and time to disease progression in the penultimate platinum-based chemotherapy prior to enrollment (6-12 month versus >12 months) as covariates.
- † The p-value is derived from a stratified log-rank test.
Lynparza tablets
(n=196)
Placebo
(n=99)
Progression-Free Survival
Number of events (%)
107 (54.6%)
80 (80.8%)
Median, months
19.1
5.5
Hazard ratio* (95% CI)
0.30 (0.22, 0.41)
p-value†
<0.0001
Figure 2 Kaplan-Meier Curves of Investigator-Assessed Progression-Free Survival – SOLO-2
Study 19
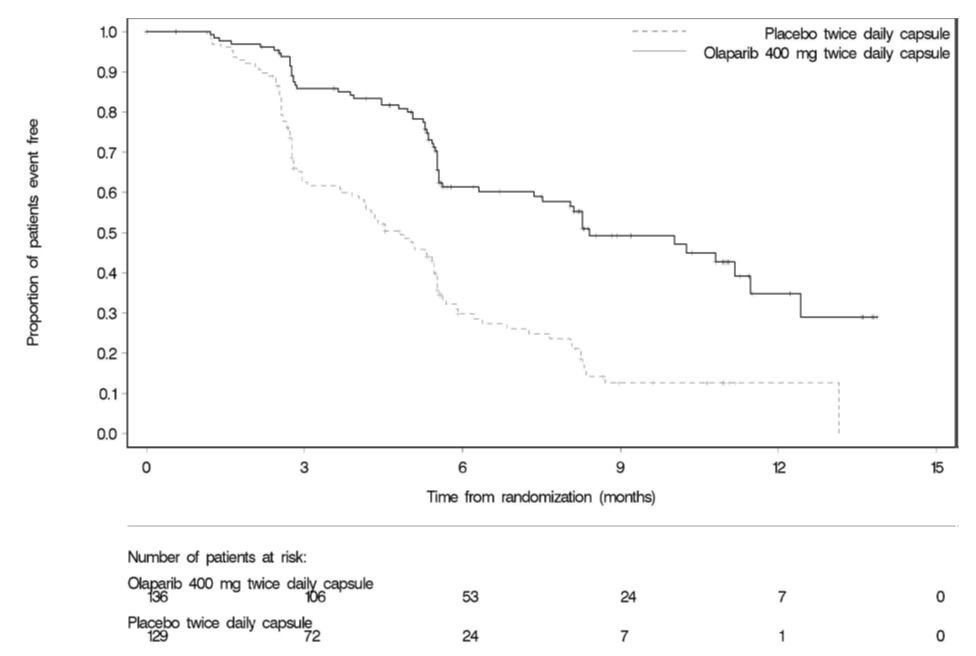
The efficacy of Lynparza was evaluated in Study 19 (NCT00753545), a randomized (1:1) double-blind, placebo-controlled trial in patients with platinum-sensitive ovarian cancer who had received 2 or more previous platinum-containing regimens. Patients were randomized to Lynparza capsules 400 mg orally twice daily or placebo until unacceptable toxicity or progressive disease. Randomization was stratified by response to last platinum chemotherapy (complete response versus partial response), time to disease progression in the penultimate platinum-based chemotherapy (6-12 months versus >12 months), and descent (Jewish versus non-Jewish). The major efficacy outcome measure was investigator-assessed PFS according to RECIST, version 1.0.
A total of 265 patients were randomized, 136 to Lynparza and 129 to placebo. The median age of patients treated with Lynparza was 58 years (range: 21 to 89) and 59 years (range 33 to 84) among patients treated with placebo. ECOG PS was 0 in 81% of patients receiving Lynparza and 74% of patients receiving placebo. Of all patients, 97% were White, 19% were enrolled in the US or Canada, 45% were in complete response following their most recent platinum chemotherapy regimen, and 40% had a progression-free interval of 6-12 months since their penultimate platinum. Prior bevacizumab therapy was reported for 13% of patients receiving Lynparza and 16% of patients receiving placebo.
A retrospective analysis for germline BRCA mutation status, some performed using the Myriad test, indicated that 36% (n=96) of patients from the ITT population had deleterious gBRCA mutation, including 39% (n=53) of patients on Lynparza and 33% (n=43) of patients on placebo. Efficacy results are presented in Table 15 and Figure 3. Study 19 demonstrated a statistically significant improvement in investigator-assessed PFS in patients treated with Lynparza versus placebo.
Table 15 Efficacy Results - Study 19 (Investigator Assessment) - * Hazard ratio from a Cox proportional hazards model including response to last platinum chemotherapy (complete response versus partial response), time to disease progression in the penultimate platinum-based chemotherapy (6-12 months versus >12 months) and Jewish descent (yes versus no) as covariates.
- † The p-value is derived from a Cox proportional hazards model.
- ‡ Without adjusting for multiple analyses.
Lynparza capsules
(n=136)
Placebo
(n=129)
Progression-Free Survival
Number of events (%)
60 (44%)
94 (73%)
Median, months
8.4
4.8
Hazard ratio*(95% CI)
0.35 (0.25, 0.49)
p-value†
<0.0001
Overall Survival‡
Number of events (%)
98 (72%)
112 (87%)
Median, months
29.8
27.8
Hazard ratio (95% CI)
0.73 (0.55, 0.95)
Figure 3 Kaplan-Meier Curves of Investigator-Assessed Progression-Free Survival – Study 19
14.3 Advanced Germline BRCA-mutated Ovarian Cancer Treated with 3 or More Prior Lines of Chemotherapy
The efficacy of Lynparza was investigated in a single-arm study of patients with deleterious or suspected deleterious gBRCAm advanced cancers. A total of 137 patients with measurable, advanced gBRCAm ovarian cancer treated with three or more prior lines of chemotherapy were enrolled. All patients received Lynparza capsules 400 mg orally twice daily until disease progression or intolerable toxicity. The efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) as assessed by the investigator according to RECIST, version 1.0.
The median age of the patients was 58 years, the majority were White (94%) and 93% had an ECOG PS of 0 or 1. Deleterious or suspected deleterious gBRCAm status was verified retrospectively in 97% (59/61) of the patients for whom blood samples were available by the BRACAnalysis CDxTM.
Efficacy results are summarized in Table 16.
Table 16 Overall Response and Duration of Response in Patients with gBRCA-mutated Advanced Ovarian Cancer Who Received 3 or More Lines of Chemotherapy Lynparza Capsules
n=137
Objective Response Rate (95% CI)
34% (26, 42)
Complete response
2%
Partial response
32%
Median DOR in months (95% CI)
7.9 (5.6, 9.6)
14.4 Treatment of Germline BRCA-mutated HER2-negative Metastatic Breast Cancer
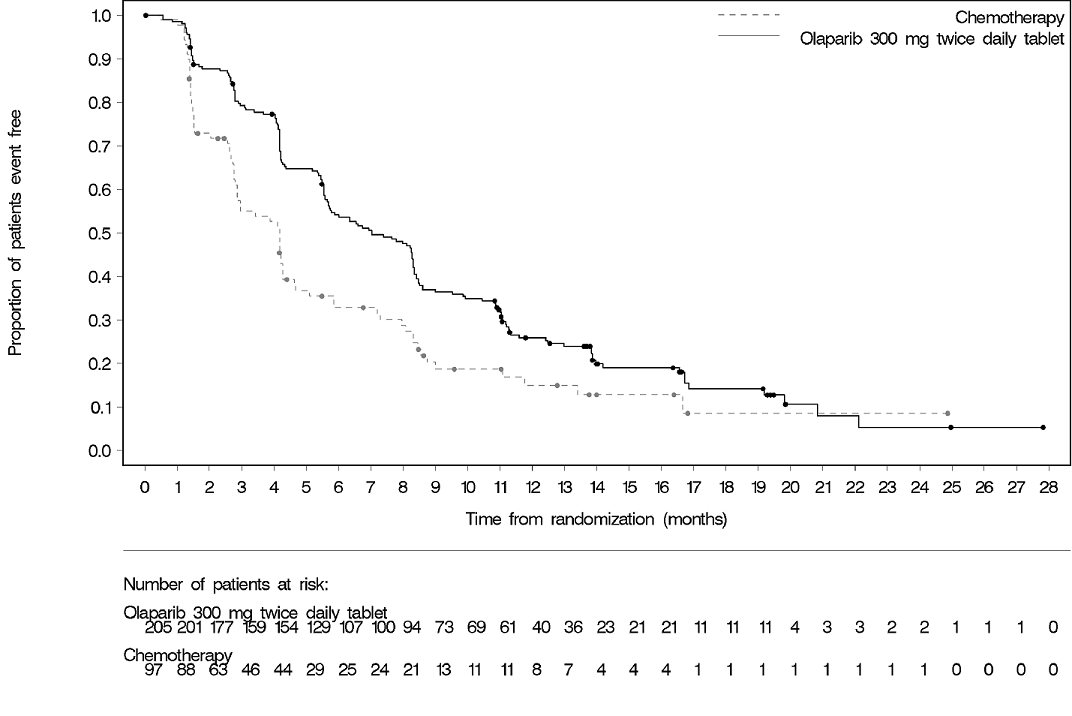
The efficacy of Lynparza was evaluated in OlympiAD (NCT02000622), an open-label randomized (2:1) study in patients with gBRCAm HER2-negative metastatic breast cancer. Patients were required to have received treatment with an anthracycline (unless contraindicated) and a taxane, in the neoadjuvant, adjuvant or metastatic setting. Patients with hormone receptor-positive disease must have progressed on at least 1 endocrine therapy (adjuvant or metastatic), or have disease that the treating healthcare provider believed to be inappropriate for endocrine therapy. Patients with prior platinum therapy were required to have no evidence of disease progress during platinum treatment. No prior treatment with a PARP inhibitor was permitted. Patients were randomized to Lynparza tablets 300 mg orally twice daily or healthcare provider’s choice of chemotherapy (capecitabine, eribulin, or vinorelbine, at standard doses) until progression or unacceptable toxicity. Randomization was stratified by prior use of chemotherapy for metastatic disease (yes vs no), hormone receptor status (hormone receptor positive vs triple negative), and previous use of platinum-based chemotherapy (yes vs no). The major efficacy outcome measure was PFS assessed by blinded independent central review (BICR) using RECIST version 1.1.
A total of 302 patients were randomized, 205 to Lynparza and 97 to chemotherapy. Among the 205 patients treated with Lynparza, the median age was 44 years (range: 22 to 76), 65% were White, 4% were males and all the patients had an ECOG PS of 0 or 1. Approximately 50% of patients had triple-negative tumors and 50% had estrogen receptor and/or progesterone receptor positive tumors and the proportions were balanced across treatment arms. Patients in each treatment arm had received a median of 1 prior chemotherapy regimen for metastatic disease; approximately 30% had not received a prior chemotherapy regimen for metastatic breast cancer. Twenty-one percent of patients in the Lynparza arm and 14% in the chemotherapy arm had received platinum therapy for metastatic disease. Seven percent of patients in each treatment arm had received platinum therapy for localized disease.
Of the 302 patients randomized onto OlympiAD, 299 were tested with the BRACAnalysis CDx® and 297 were confirmed to have deleterious or suspected deleterious gBRCAm status; 202 were randomized to the Lynparza arm and 95 to the healthcare provider’s choice of chemotherapy arm.
A statistically significant improvement in PFS was demonstrated for the Lynparza arm compared to the chemotherapy arm. Efficacy data for OlympiAD are displayed in Table 17 and Figure 4. Consistent results were observed across patient subgroups defined by study stratification factors. An exploratory analysis of investigator-assessed PFS was consistent with the BICR-assessed PFS results.
Table 17 Efficacy Results - OlympiAD (BICR-assessed) - * Hazard ratio is derived from a stratified log-rank test, stratified by ER, PgR negative versus ER and or PgR positive and prior chemotherapy (yes versus no).
- † For PFS, p-value (2-sided) was compared to 0.05.
- ‡ Response based on confirmed responses. The confirmed complete response rate was 7.8% for Lynparza compared to 1.5% for chemotherapy arm.
Lynparza tablets
(n=205)
Chemotherapy
(n=97)
Progression-Free Survival
Number of events (%)
163 (80%)
71 (73%)
Median, months
7.0
4.2
Hazard ratio (95% CI)*
0.58 (0.43, 0.80)
p-value†
0.0009
Patients with Measurable Disease
n=167
n=66
Objective Response Rate (95% CI)‡
52% (44, 60)
23% (13, 35)
Overall Survival
Number of events (%)
130 (63%)
62 (64%)
Median, months
19.3
17.1
Hazard ratio (95% CI)*
0.90 (0.66, 1.23)
Figure 4 Kaplan-Meier Curves of Progression-Free Survival – OlympiAD
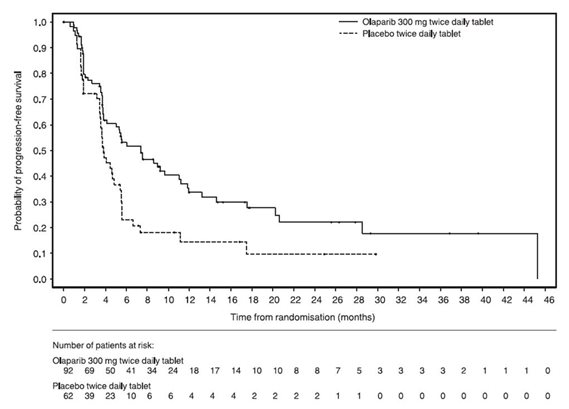
14.5 First-Line Maintenance Treatment of Germline BRCA-mutated Metastatic Pancreatic Adenocarcinoma
The efficacy of Lynparza was evaluated in POLO (NCT02184195), a randomized (3:2), double-blind placebo-controlled, multi-center trial. Patients were required to have metastatic pancreatic adenocarcinoma with a deleterious or suspected deleterious germline BRCA mutation (gBRCAm) and absence of disease progression after receipt of first-line platinum-based chemotherapy for at least 16 weeks. Patients were randomized to receive Lynparza tablets 300 mg orally twice daily or placebo until disease progression or unacceptable toxicity. The major efficacy outcome measure was PFS by BICR using RECIST, version 1.1 modified to assess patients with clinical complete response at entry who were assessed as having no evidence of disease unless they had progressed based on the appearance of new lesions. Additional efficacy outcome measures were OS and ORR.
A total of 154 patients were randomized, 92 to Lynparza and 62 to placebo. The median age was 57 years (range 36 to 84); 54% were male; 92% were White, 4% were Asian and 3% were Black; baseline ECOG PS was 0 (67%) or 1 (31%). The median time from initiation of first-line platinum-based chemotherapy to randomization was 5.8 months (range 3.4 to 33.4 months). Seventy-five percent (75%) of patients received FOLFIRINOX with a median of 9 cycles (range 4-61), 8% received FOLFOX or XELOX, 4% received GEMOX, and 3% received gemcitabine plus cisplatin; 49% achieved a complete or partial response to platinum-based chemotherapy.
All patients had a deleterious or suspected deleterious germline BRCA-mutation as detected by the Myriad BRACAnalysis® or BRACAnalysis CDx® at a central laboratory only (n=106), local BRCA test only (n=4), or both local and central testing (n=44). Among the 150 patients with central test results, 30% had a mutation in BRCA1; 69% had a mutation in BRCA2; and 1 patient (1%) had mutations in both BRCA1 and BRCA2.
Efficacy results of POLO are provided in Table 18 and Figure 5.
Table 18 Efficacy Results - POLO (BICR-assessed) - * Number of events: Progression - Lynparza 55, placebo 44; death before BICR-documented progression - Lynparza 5, placebo 0
- † Hazard ratio, 95% CI, and p-value calculated from a log-rank test. A hazard ratio <1 favors Lynparza.
Lynparza tablets
(n=92)Placebo
(n=62)Progression-Free Survival
- Number of events (%)*
60 (65%)
44 (71%)
Median, months (95% CI)
7.4 (4.1, 11.0)
3.8 (3.5, 4.9)
Hazard ratio† (95% CI)
0.53 (0.35, 0.81)
p-value
0.0035
Patients with Measurable Disease
n=78
n=52
Objective Response Rate (95% CI)
23% (14, 34)
12% (4, 23)
Complete response (%)
2 (2.6)
0
Partial response (%)
16 (21)
6 (12)
Duration of Response (DOR)
- Median time in months (95% CI)
25 (15, NC)
- 4 (2, NC)
NC Not calculable
The result of an OS interim analysis conducted based on 67% information fraction did not show a statistically significant improvement in OS for Lynparza compared to placebo.
Figure 5 Kaplan-Meier Curves of BICR-Assessed Progression-Free Survival-POLO
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Lynparza is available as 150 mg and 100 mg tablets.
-
150 mg tablets: green to green/grey, oval, bi-convex, film-coated tablet, with debossment ‘OP150’ on one side and plain on the reverse, are available in:
- ∘ Bottles of 60 tablets (NDC: 0310-0679-60) and
- ∘ Bottles of 120 tablets (NDC: 0310-0679-12).
-
100 mg tablets: yellow to dark yellow, oval, bi-convex, film-coated tablet, with debossment ‘OP100’ on one side and plain on the reverse, are available in:
- ∘ Bottles of 60 tablets (NDC: 0310-0668-60) and
- ∘ Bottles of 120 tablets (NDC: 0310-0668-12).
Store at 20ºC to 25ºC (68ºF to 77ºF), excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Store in original bottle to protect from moisture.
-
150 mg tablets: green to green/grey, oval, bi-convex, film-coated tablet, with debossment ‘OP150’ on one side and plain on the reverse, are available in:
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
MDS/AML
Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. This may be a sign of hematological toxicity or a more serious uncommon bone marrow problem called ‘myelodysplastic syndrome’ (MDS) or ‘acute myeloid leukemia’ (AML) which have been reported in patients treated with Lynparza [see Warnings and Precautions (5.1)].
Pneumonitis
Advise patients to contact their healthcare provider if they experience any new or worsening respiratory symptoms including shortness of breath, fever, cough, or wheezing [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
Inform pregnant women of the risk to a fetus and potential loss of the pregnancy. Advise females to inform their healthcare provider of known or suspected pregnancy [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with Lynparza and for 6 months after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for 3 months after receiving the last dose of Lynparza. Advise male patients not to donate sperm during therapy and for 3 months following the last dose of Lynparza [see Warnings and Precautions (5.3) and Use in Specific Population (8.3)].
Lactation
Advise patients not to breastfeed while taking Lynparza and for one month after receiving the last dose [see Use in Specific Populations (8.2)].
Drug Interactions
Advise patients and caregivers to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products. Inform patients to avoid grapefruit, grapefruit juice, Seville oranges, and Seville orange juice while taking Lynparza [see Drug Interactions (7.2)].
Nausea/Vomiting
Advise patients that mild or moderate nausea and/or vomiting is very common in patients receiving Lynparza and that they should contact their healthcare provider who will advise on available antiemetic treatment options [see Adverse Reactions (6.1)].
- Distributed by:
- AstraZeneca Pharmaceuticals LP
- Wilmington, DE 19850
- © AstraZeneca 2019
-
MEDICATION GUIDE
Medication Guide
Lynparza® (Lin-par-zah)
(olaparib)
tablets
What is the most important information I should know about Lynparza?
Lynparza may cause serious side effects, including:
Bone marrow problems called Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML). Some people who have ovarian cancer or breast cancer and who have received previous treatment with chemotherapy, radiotherapy or certain other medicines for their cancer have developed MDS or AML during treatment with Lynparza. MDS or AML may lead to death. If you develop MDS or AML, your healthcare provider will stop treatment with Lynparza.
Symptoms of low blood cell counts are common during treatment with Lynparza, but can be a sign of serious bone marrow problems, including MDS or AML. Symptoms may include:
- weakness
- weight loss
- fever
- frequent infections
- blood in urine or stool
- shortness of breath
- feeling very tired
- bruising or bleeding more easily
Your healthcare provider will do blood tests to check your blood cell counts:
- before treatment with Lynparza
- every month during treatment with Lynparza
- weekly if you have low blood cell counts that last a long time. Your healthcare provider may stop treatment with Lynparza until your blood cell counts improve.
Lung problems (pneumonitis). Tell your healthcare provider if you have any new or worsening symptoms of lung problems, including shortness of breath, fever, cough, or wheezing. Your healthcare provider may do a chest x-ray if you have any of these symptoms. Your healthcare provider may temporarily or completely stop treatment if you develop pneumonitis. Pneumonitis may lead to death.
What is Lynparza?
Lynparza is a prescription medicine used to treat adults who have:
- advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer with a certain type of inherited (germline) or acquired (somatic) abnormal BRCA gene. Lynparza is used as maintenance treatment after the cancer has responded to your first treatment with platinum-based chemotherapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- ovarian cancer, fallopian tube cancer, or primary peritoneal cancer, as maintenance treatment, when the cancer has come back. Lynparza is used after the cancer has responded to treatment with platinum-based chemotherapy.
- advanced ovarian cancer with a certain type of abnormal inherited BRCA gene, and have received treatment with 3 or more prior types of chemotherapy medicines. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- a certain type of abnormal inherited BRCA gene, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body (metastatic). You should have received chemotherapy medicines, either before or after your cancer has spread. If you have hormone receptor (HR)-positive disease, you should have been treated with hormonal therapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- metastatic pancreatic cancer with a certain type of abnormal inherited BRCA gene. Lynparza is used as maintenance treatment after your cancer has not progressed on at least 16 weeks of treatment with platinum-based chemotherapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
It is not known if Lynparza is safe and effective in children.
Before taking Lynparza, tell your healthcare provider about all of your medical conditions, including if you:
- have lung or breathing problems
- have kidney problems
-
are pregnant, become pregnant, or plan to become pregnant. Lynparza can harm your unborn baby and may cause loss of pregnancy (miscarriage).
- ∘ If you are able to become pregnant, your healthcare provider may do a pregnancy test before you start treatment with Lynparza
- ∘ Females who are able to become pregnant should use effective birth control (contraception) during treatment with Lynparza and for 6 months after the last dose of Lynparza. Talk to your healthcare provider about birth control methods that may be right for you. Tell your healthcare provider right away if you become pregnant or think you might be pregnant following treatment with Lynparza.
- ∘ Males with female partners who are pregnant or able to become pregnant should use effective birth control (contraception) during treatment with Lynparza and for 3 months after the last dose of Lynparza.
- ∘ Do not donate sperm during treatment with Lynparza and for 3 months after your final dose.
- are breastfeeding or plan to breastfeed. It is not known if Lynparza passes into your breast milk. Do not breastfeed during treatment with Lynparza and for 1 month after receiving the last dose of Lynparza. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Lynparza and certain other medicines may affect how Lynparza works and may cause side effects.
How should I take Lynparza?
- Take Lynparza tablets exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Lynparza unless your healthcare provider tells you to. Your healthcare provider may temporarily stop treatment with Lynparza or change your dose of Lynparza if you experience side effects.
- Your healthcare provider will decide how long you stay on treatment.
- Do not take more than 4 Lynparza tablets in 1 day. If you have any questions about Lynparza, please talk to your healthcare provider or pharmacist.
- Take Lynparza by mouth 2 times a day.
- Each dose should be taken about 12 hours apart.
- Swallow Lynparza tablets whole. Do not chew, crush, dissolve, or divide the tablets.
- Take Lynparza with or without food.
- If you miss a dose of Lynparza, take your next dose at your usual scheduled time. Do not take an extra dose to make up for a missed dose.
- If you take too much Lynparza, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Lynparza?
Avoid grapefruit, grapefruit juice, Seville oranges and Seville orange juice during treatment with Lynparza since they may increase the level of Lynparza in your blood.
What are the possible side effects of Lynparza?
Lynparza may cause serious side effects.
See “What is the most important information I should know about Lynparza?”
The most common side effects of Lynparza are:
- nausea or vomiting. Tell your healthcare provider if you get nausea or vomiting. Your healthcare provider may prescribe medicines to treat these symptoms.
- ∘ low number of red or white blood cells
- ∘ stomach-area (abdominal) pain
- ∘ dizziness
- ∘ tiredness or weakness
- ∘ sore throat or runny nose
- ∘ diarrhea
- ∘ joint, muscle, and back pain
- ∘ headache
- ∘ constipation
- ∘ mouth sores
- ∘ respiratory tract infections
- ∘ changes in kidney function blood test
- ∘ changes in the way food tastes
- ∘ loss of appetite
- ∘ low number of platelets
- ∘ indigestion or heartburn
These are not all of the possible side effects of Lynparza.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Lynparza?
- Store Lynparza at room temperature, between 68°F to 77°F (20°C to 25°C).
- Store Lynparza in the original bottle to protect it from moisture.
Keep Lynparza and all medicines out of the reach of children.
General information about the safe and effective use of Lynparza.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Lynparza for a condition for which it was not prescribed. Do not give Lynparza to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Lynparza that is written for health professionals.
What are the ingredients in Lynparza?
Active ingredient: olaparib
Inactive ingredients:
Tablet contains: copovidone, mannitol, colloidal silicon dioxide and sodium stearyl fumarate
Tablet coating contains: hypromellose, polyethylene glycol 400, titanium dioxide, ferric oxide yellow and ferrosoferric oxide (150 mg tablet only)
Lynparza is a registered trademark of the AstraZeneca group of companies.
© AstraZeneca 2019
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
For more information, call 1-800-236-9933 or go to www.Lynparza.com.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 12/2019
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 100 mg
NDC: 0310-0668-60
Lynparza®
(olaparib) tablets
100 mg
Dispense accompanying
Medication Guide to each patient.
60 Tablets
Rx only
AstraZeneca
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 150 mg
NDC: 0310-0679-12
Lynparza®
(olaparib) tablets
150 mg
Dispense accompanying
Medication Guide to each patient.
120 Tablets
Rx only
AstraZeneca
-
INGREDIENTS AND APPEARANCE
LYNPARZA
olaparib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-0668 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLAPARIB (UNII: WOH1JD9AR8) (OLAPARIB - UNII:WOH1JD9AR8) OLAPARIB 100 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) WATER (UNII: 059QF0KO0R) FERROSOFERRIC OXIDE (UNII: XM0M87F357) COPOVIDONE K25-31 (UNII: D9C330MD8B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MANNITOL (UNII: 3OWL53L36A) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color YELLOW (opaque) Score no score Shape OVAL (biconvex) Size 14mm Flavor Imprint Code OP;100;plain Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-0668-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2017 2 NDC: 0310-0668-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208558 08/17/2017 LYNPARZA
olaparib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-0679 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLAPARIB (UNII: WOH1JD9AR8) (OLAPARIB - UNII:WOH1JD9AR8) OLAPARIB 150 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) COPOVIDONE K25-31 (UNII: D9C330MD8B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) MANNITOL (UNII: 3OWL53L36A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color GREEN (grey) Score no score Shape OVAL (bi-convex) Size 14mm Flavor Imprint Code OP150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-0679-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2017 2 NDC: 0310-0679-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2017 3 NDC: 0310-0679-95 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208558 08/17/2017 Labeler - AstraZeneca Pharmaceuticals LP (054743190) Registrant - AstraZeneca PLC (230790719)
Trademark Results [Lynparza]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LYNPARZA 86476170 4991136 Live/Registered |
AstraZeneca AB 2014-12-10 |
 LYNPARZA 86190624 4903563 Live/Registered |
AstraZeneca AB 2014-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.