CALCIUM DISODIUM VERSENATE- edetate calcium disodium injection
Calcium Disodium Versenate by
Drug Labeling and Warnings
Calcium Disodium Versenate by is a Prescription medication manufactured, distributed, or labeled by Bausch Health Us, LLC, CP Pharmaceuticals Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNINGS
Calcium Disodium Versenate is capable of producing toxic effects which can be fatal. Lead encephalopathy is relatively rare in adults, but occurs more often in pediatric patients in whom it may be incipient and thus overlooked. The mortality rate in pediatric patients has been high. Patients with lead encephalopathy and cerebral edema may experience a lethal increase in intracranial pressure following intravenous infusion; the intramuscular route is preferred for these patients. In cases where the intravenous route is necessary, avoid rapid infusion. The dosage schedule should be followed and at no time should the recommended daily dose be exceeded.
-
DESCRIPTION
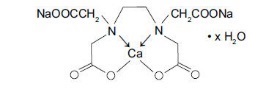
Calcium Disodium Versenate (edetate calcium disodium injection, USP) is a sterile, injectable, chelating agent in concentrated solution for intravenous infusion or intramuscular injection. Each 5 ml ampul contains 1000 mg of edetate calcium disodium (equivalent to 200 mg/ml) in water for injection. Chemically, this product is called [[N,N'-1,2-ethanediyl-bis[N-(carboxymethyl)-glycinato]](4-)-N,N',O,O',ON,ON']-, disodium, hydrate, (OC-6-21)-Calciate(2-).
Structural Formula:
C10H12CaN2Na2O8 × H2O
Molecular weight 374.27 (anhydrous) -
CLINICAL PHARMACOLOGY
The pharmacologic effects of edetate calcium disodium are due to the formation of chelates with divalent and trivalent metals. A stable chelate will form with any metal that has the ability to displace calcium from the molecule, a feature shared by lead, zinc, cadmium, manganese, iron and mercury. The amounts of manganese and iron mobilized are not significant. Copper1 is not mobilized and mercury is unavailable for chelation because it is too tightly bound to body ligands or it is stored in inaccessible body compartments. The excretion of calcium by the body is not increased following intravenous administration of edetate calcium disodium, but the excretion of zinc is considerably increased.1
Edetate calcium disodium is poorly absorbed from the gastrointestinal tract. In blood, all the drug is found in the plasma. Edetate calcium disodium does not appear to penetrate cells; it is distributed primarily in the extracellular fluid with only about 5% of the plasma concentration found in spinal fluid.
The half life of edetate calcium disodium is 20 to 60 minutes. It is excreted primarily by the kidney, with about 50% excreted in one hour and over 95% within 24 hours.2 Almost none of the compound is metabolized.
The primary source of lead chelated by Calcium Disodium Versenate is from bone; subsequently, soft-tissue lead is redistributed to bone when chelation is stopped.3,4 There is also some reduction in kidney lead levels following chelation therapy.
It has been shown in animals that following a single dose of Calcium Disodium Versenate urinary lead output increases, blood lead concentration decreases, but brain lead is significantly increased due to internal redistribution of lead.5 (See WARNINGS.) These data are in agreement with the recent results of others in experimental animals showing that after a five day course of treatment there is no net reduction in brain lead.6
-
INDICATIONS AND USAGE
Edetate calcium disodium is indicated for the reduction of blood levels and depot stores of lead in lead poisoning (acute and chronic) and lead encephalopathy, in both pediatric populations and adults.
Chelation therapy should not replace effective measures to eliminate or reduce further exposure to lead.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General Precautions
Edetate calcium disodium may produce the same renal damage as lead poisoning, such as proteinuria and microscopic hematuria. Treatment-induced nephrotoxicity is dose-dependent and may be reduced by assuring adequate diuresis before therapy begins. Urine flow must be monitored throughout therapy which must be stopped if anuria or severe oliguria develop. The proximal tubule hydropic degeneration usually recovers upon cessation of therapy. Edetate calcium disodium must be used in reduced doses in patients with pre-existing mild renal disease. Patients should be monitored for cardiac rhythm irregularities and other ECG changes during intravenous therapy.
Information for patients
Patients should be instructed to immediately inform their physician if urine output stops for a period of 12 hours.
Laboratory tests
Urinalysis and urine sediment, renal and hepatic function and serum electrolyte levels should be checked before each course of therapy and then be monitored daily during therapy in severe cases, and in less serious cases after the second and fifth day of therapy. Therapy must be discontinued at the first sign of renal toxicity. The presence of large renal epithelial cells or increasing number of red blood cells in urinary sediment or greater proteinuria call for immediate stopping of edetate calcium disodium administration. Alkaline phosphatase values are frequently depressed (possibly due to decreased serum zinc levels), but return to normal within 48 hours after cessation of therapy. Elevated erythrocyte protoporphyrin levels (> 35 mcg/dl of whole blood) indicate the need to perform a venous blood lead determination. If the whole blood lead concentration is between 25–55 mcg/dl a mobilization test can be considered.7,8 (See Diagnostic Test.) An elevation of urinary coproporphyrin (adults: > 250 mcg/day; pediatric patients under 80 lbs: > 75 mcg/day) and elevation of urinary delta aminolevulinic acid (ALA) (adults: > 4 mg/day; pediatric patients: > 3 mg/m2/day) are associated with blood lead levels > 40 mcg/dl. Urinary coproporphyrin may be falsely negative in terminal patients and in severely iron-depleted pediatric patients who are not regenerating heme.9 In growing pediatric patients long bone x-rays showing lead lines and abdominal x-rays showing radio-opaque material in the abdomen may be of help in estimating the level of exposure to lead.
Drug Interactions
There is no known drug interference with standard clinical laboratory tests. Steroids enhance the renal toxicity of edetate calcium disodium in animals.7 Edetate calcium disodium interferes with the action of zinc insulin preparations by chelating the zinc.7
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies have not been conducted with edetate calcium disodium to evaluate its carcinogenic potential, mutagenic potential or its effect on fertility.
Pregnancy
Category B
One reproduction study was performed in rats at doses up to 13 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to Calcium Disodium Versenate.10 Another reproduction study performed in rats at doses up to about 25 to 40 times the human dose revealed evidence of fetal malformations due to Calcium Disodium Versenate, which were prevented by simultaneous supplementation of dietary zinc.11 There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
Calcium Disodium Versenate has no recognized use during labor and delivery, and its effects during these processes are unknown.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Calcium Disodium Versenate is administered to a nursing woman.
Pediatric Use
Since lead poisoning occurs in pediatric populations and adults but is frequently more severe in pediatric patients, Calcium Disodium Versenate is used in patients of all ages. The intramuscular route is preferred by some for young pediatric patients. In cases where the intravenous route is necessary, avoid rapid infusion. (See WARNINGS.) Urine flow must be monitored throughout therapy; Calcium Disodium Versenate therapy must be stopped if anuria or severe oliguria develops. (See General Precautions.) At no time should the recommended daily dosage be exceeded. (See DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONS
The following adverse effects have been associated with the use of edetate calcium disodium:
Body as a Whole: pain at intramuscular injection site, fever, chills, malaise, fatigue, myalgia, arthralgia.
Cardiovascular: hypotension, cardiac rhythm irregularities.
Renal: acute necrosis of proximal tubules (which may result in fatal nephrosis), infrequent changes in distal tubules and glomeruli.
Urinary: glycosuria, proteinuria, microscopic hematuria and large epithelial cells in urinary sediment.
Nervous System: tremors, headache, numbness, tingling.
Gastrointestinal: cheilosis, nausea, vomiting, anorexia, excessive thirst.
Hepatic: mild increases in SGOT and SGPT are common, and return to normal within 48 hours after cessation of therapy.
Immunogenic: histamine-like reactions (sneezing, nasal congestion, lacrimation), rash.
Hematopoietic: transient bone marrow depression, anemia.
Metabolic: zinc deficiency, hypercalcemia.
-
OVERDOSAGE
Symptoms
Inadvertent administration of 5 times the recommended dose, infused intravenously over a 24 hour period, to an asymptomatic 16 month old patient with a blood lead content of 56 mcg/dl did not cause any ill effects. Edetate calcium disodium can aggravate the symptoms of severe lead poisoning, therefore, most toxic effects (cerebral edema, renal tubular necrosis) appear to be associated with lead poisoning. Because of cerebral edema, a therapeutic dose may be lethal to an adult or a pediatric patient with lead encephalopathy. Higher dosage of edetate calcium disodium may produce a more severe zinc deficiency.
Treatment
Cerebral edema should be treated with repeated doses of mannitol. Steroids enhance the renal toxicity of edetate calcium disodium in animals and, therefore, are no longer recommended.7 Zinc levels must be monitored. Good urinary output must be maintained because diuresis will enhance drug elimination. It is not known if edetate calcium disodium is dialyzable.
-
DOSAGE AND ADMINISTRATION
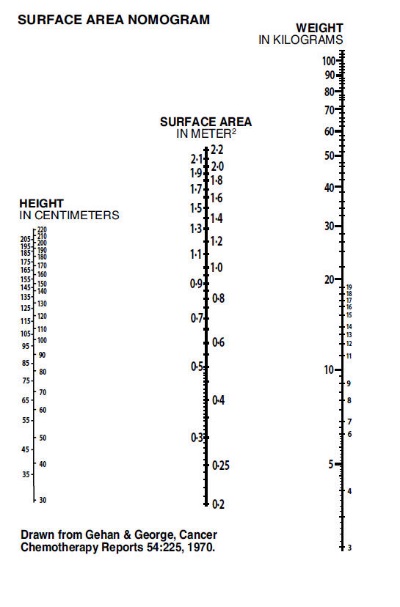
When a source for the lead intoxication has been identified, the patient should be removed from the source, if possible. The recommended dose of Calcium Disodium Versenate for asymptomatic adults and pediatric patients whose blood lead level is < 70 mcg/dl but > 20 mcg/dl (World Health Organization recommended upper allowable level) is 1000 mg/m2/day whether given intravenously or intramuscularly. (See Surface Area Nomogram.)
For adults with lead nephropathy, the following dosing regimen has been suggested: 500 mg/m2 every 24 hours for 5 days for patients with serum creatinine levels of 2–3 mg/dl, every 48 hours for 3 doses for patients with creatinine levels of 3–4 mg/dl, and once weekly for patients with creatinine levels above 4 mg/dl. These regimens may be repeated at one month intervals.12
Calcium Disodium Versenate, used alone, may aggravate symptoms in patients with very high blood lead levels. When the blood lead level is > 70 mcg/dl or clinical symptoms consistent with lead poisoning are present, it is recommended that Calcium Disodium Versenate be used in conjunction with BAL (dimercaprol). Please consult published protocols and specialized references for dosage recommendations of combination therapy.14–18
Therapy of lead poisoning in adults and pediatric patients with Calcium Disodium Versenate is continued over a period of five days. Therapy is then interrupted for 2 to 4 days to allow redistribution of the lead and to prevent severe depletion of zinc and other essential metals. Two courses of treatment are usually employed; however, it depends on severity of the lead toxicity and the patient's tolerance of the drug.
Calcium Disodium Versenate is equally effective whether administered intravenously or intramuscularly. The intramuscular route is used for all patients with overt lead encephalopathy and this route is preferred by some for young pediatric patients.
Acutely ill individuals may be dehydrated from vomiting. Since edetate calcium disodium is excreted almost exclusively in the urine, it is very important to establish urine flow with intravenous fluid administration before the first dose of the chelating agent is given; however, excessive fluid must be avoided in patients with encephalopathy. Once urine flow is established, further intravenous fluid is restricted to basal water and electrolyte requirements. Administration of Calcium Disodium Versenate should be stopped whenever there is cessation of urine flow in order to avoid unduly high tissue levels of the drug. Edetate calcium disodium must be used in reduced doses in patients with pre-existing mild renal disease.
Intravenous Administration
Add the total daily dose of Calcium Disodium Versenate (1000 mg/m2/day) to 250–500 ml of 5% dextrose or 0.9% sodium chloride injection. The total daily dose should be infused over a period of 8–12 hours. Calcium Disodium Versenate injection is incompatible with 10% dextrose, 10% invert sugar in 0.9% sodium chloride, lactate Ringer's, Ringer's, one-sixth molar sodium lactate injections, and with injectable amphotericin B and hydralazine hydrochloride.
Intramuscular Administration
The total daily dosage (1000 mg/m2/day) should be divided into equal doses spaced 8–12 hours apart. Lidocaine or procaine should be added to the Calcium Disodium Versenate injection to minimize pain at the injection site. The final lidocaine or procaine concentration of 5 mg/ml (0.5%) can be obtained as follows: 0.25 ml of 10% lidocaine solution per 5 ml concentrated Calcium Disodium Versenate; 1 ml of 1% lidocaine or procaine solution per ml of concentrated Calcium Disodium Versenate. When used alone, regardless of method of administration, Calcium Disodium Versenate should not be given at doses larger than those recommended.
Diagnostic Test
Several methods have been described for lead mobilization tests using edetate calcium disodium to assess body stores.7, 9,12,13,18
These procedures have advantages and disadvantages that should be reviewed in current references. Edetate calcium disodium mobilization tests should not be performed in symptomatic patients and in patients with blood lead levels above 55 mcg/dl for whom appropriate therapy is indicated.
Parenteral drugs should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
REFERENCES
- Thomas DJ, Chisolm JJ. Lead, zinc and copper decorporation during calcium disodium ethylenediamine tetraacetate treatment of lead-poisoned children. J Pharmacol Exp Therapeu 1986; 239: 829–835.
- The Pharmacological Basis of Therapeutics, 7th edition, Goodman and Gilman, editors. MacMillan Publishing Company, New York, 1985, pp. 1619–1622.
- Hammond PB, Aronson AL, Olson WC. The mechanism of mobilization of lead by ethylenediaminetetraacetate. J Pharmacol Exp Therapeu 1967; 157: 196–206.
- Van deVyver FL, D'Haese PC, Visser WJ, et al. Bone lead in dialysis patients. Kidney Intl 1988; 33: 601–607.
- Cory-Slecta DA, Weiss B, Cox C. Mobilization and redistribution of lead over the course of calcium disodium ethylenediamine tetraacetate chelation therapy. J Pharmacol Exp Therapeu 1987; 243: 804–813.
- Chisolm JJ. Mobilization of lead by calcium disodium edetate. Am J Dis Child 1987; 141: 1256–1257.
- Drug Evaluations, 6th Edition, American Medical Association, Saunders, Philadelphia, 1986, pp. 1637–1639.
- Centers for Disease Control: Preventing lead poisoning in young children. Atlanta, GA, Department of Health and Human Services, 1985 Jan.
- Finberg L, Rajagopal V. Diagnosis and treatment of lead poisoning in children. J Family Med 1985 April: 3–12.
- Schardein JL, Sakowski R, Petrere J, et al. Teratogenesis studies with EDTA and its salts in rats. Toxicol Appl Pharmacol 1981; 61: 423–428.
- Swenerton H, Hurley LS. Teratogenic effects of a chelating agent and their prevention by zinc. Science 1971; 173: 62–64.
- American Hospital Formulary Service, Drug Information, 1988, pp. 1695–1698.
- Markowitz ME, Rosen JF. Assessment of lead stores in children: Validation of an 8-hour CaNa2EDTA (Calcium Disodium Versenate) provocative test. J Pediatrics 1984; 104: 337–341.
- Piomelli S, Rosen JF, Chisolm JJ, et al. Management of childhood lead poisoning. J Pediatrics 1984; 105: 523–532.
- Sachs HK, Blanksma LA, Murray EF, et al. Ambulatory treatment of lead poisoning: Report of 1,155 cases. Pediatrics 1970; 46: 389.
- Chisolm JJ. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatrics 1968; 73: 1.
- Coffin R, Phillips JL, Staples WI, et al. Treatment of lead encephalopathy in children. J Pediatrics 1966; 69: 198–206.
- Chisolm JJ. Increased lead absorption and acute lead poisoning. Current Pediatric Therapy 12, Gillis and Kagan, editors, WB Saunders, Philadelphia, 1986, pp. 667–671.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 5 mL Ampule Carton
-
INGREDIENTS AND APPEARANCE
CALCIUM DISODIUM VERSENATE
edetate calcium disodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 99207-240 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) (EDETIC ACID - UNII:9G34HU7RV0) EDETATE CALCIUM DISODIUM 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 99207-240-05 5 in 1 CARTON 06/21/2016 1 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA008922 06/21/2013 Labeler - Bausch Health Us, LLC (831922468) Establishment Name Address ID/FEI Business Operations CP Pharmaceuticals Ltd 226503308 MANUFACTURE(99207-240)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.