ALLERGY- diphenhydramine hcl tablet, film coated
Allergy by
Drug Labeling and Warnings
Allergy by is a Otc medication manufactured, distributed, or labeled by GREENBRIER INTERNATIONAL, INC., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
ASSURED™
COMPARE TO ACTIVE INGREDIENT OF
BENADRYL® ALLERGY ULTRATAB® TABLETS*Allergy
Diphenhydramine HCl 25 mg
AntihistamineSneezing, Runny Nose, Itchy Throat,
Itchy, Watery EyesActual Size
36 tablets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed
by Johnson & Johnson Corporation, owner of
the registered trademark Benadryl® Allergy
ULTRATAB® Tablets.
50844 REV1016D32907Distributed by:
Greenbrier International, Inc.
Chesapeake, VA 23320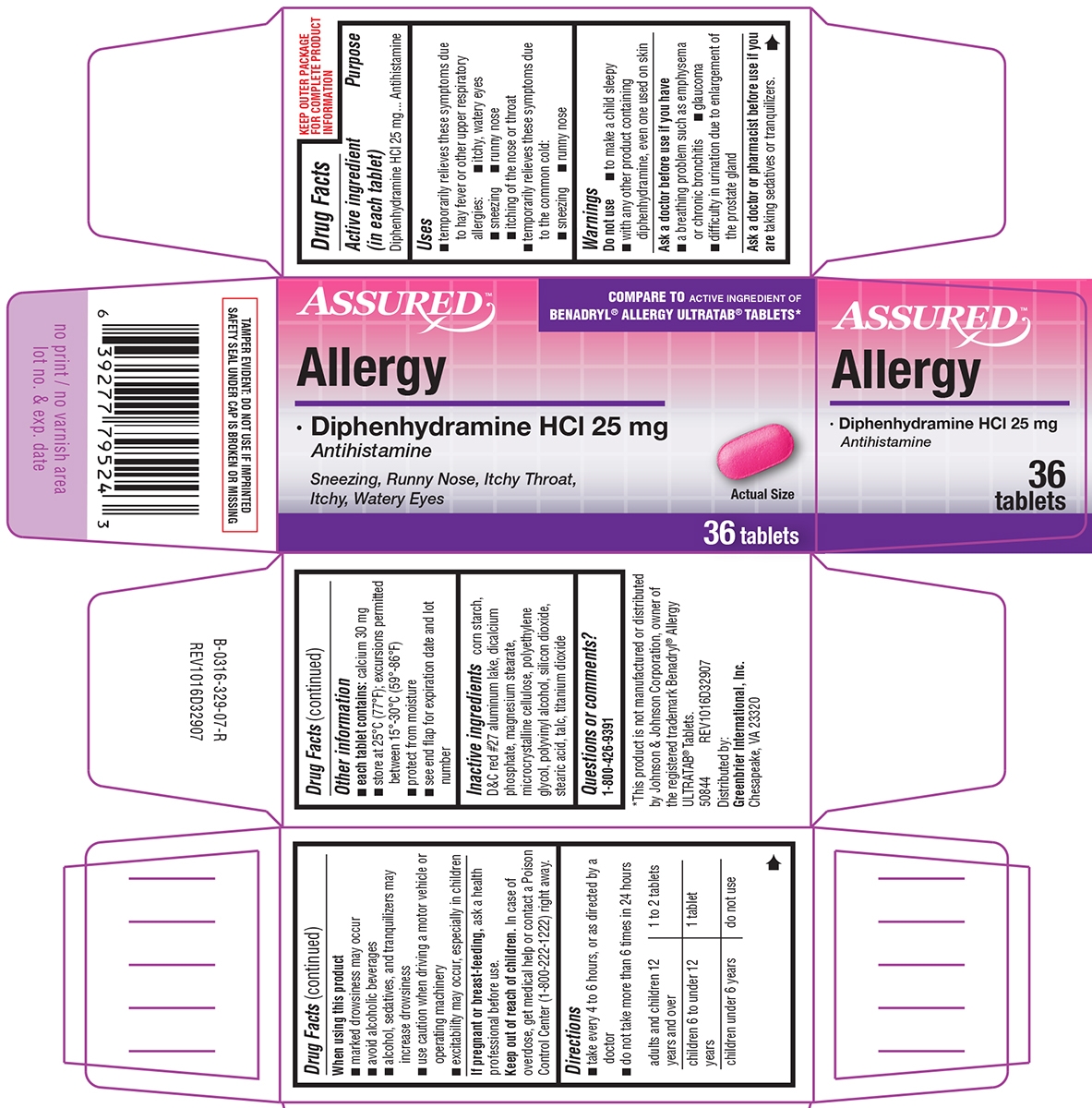
Assured 44-329
-
INGREDIENTS AND APPEARANCE
ALLERGY
diphenhydramine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 33992-0329 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) Product Characteristics Color PINK Score no score Shape OVAL Size 11mm Flavor Imprint Code 44;329 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33992-0329-7 1 in 1 CARTON 03/02/1990 1 36 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 33992-0329-1 1 in 1 CARTON 03/02/1990 2 45 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 03/02/1990 Labeler - GREENBRIER INTERNATIONAL, INC. (610322518) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(33992-0329) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(33992-0329) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 MANUFACTURE(33992-0329) , PACK(33992-0329) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(33992-0329) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(33992-0329)
Trademark Results [Allergy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY 97069656 not registered Live/Pending |
Rodriguez, Kent J 2021-10-12 |
 ALLERGY 90819383 not registered Live/Pending |
Rodriguez, Kent J 2021-07-09 |
 ALLERGY 87534443 5406627 Live/Registered |
RSM Medical Inc. 2017-07-19 |
 ALLERGY 87518600 5400551 Live/Registered |
RSM Medical Inc. 2017-07-06 |
 ALLERGY 74392251 not registered Dead/Abandoned |
Danta, Inc. 1993-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.