IBUPROFEN by WALGREENS / TIME CAP LABORATORIES, INC / MARKSANS PHARMA LIMITED IBUPROFEN tablet
IBUPROFEN by
Drug Labeling and Warnings
IBUPROFEN by is a Otc medication manufactured, distributed, or labeled by WALGREENS, TIME CAP LABORATORIES, INC, MARKSANS PHARMA LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
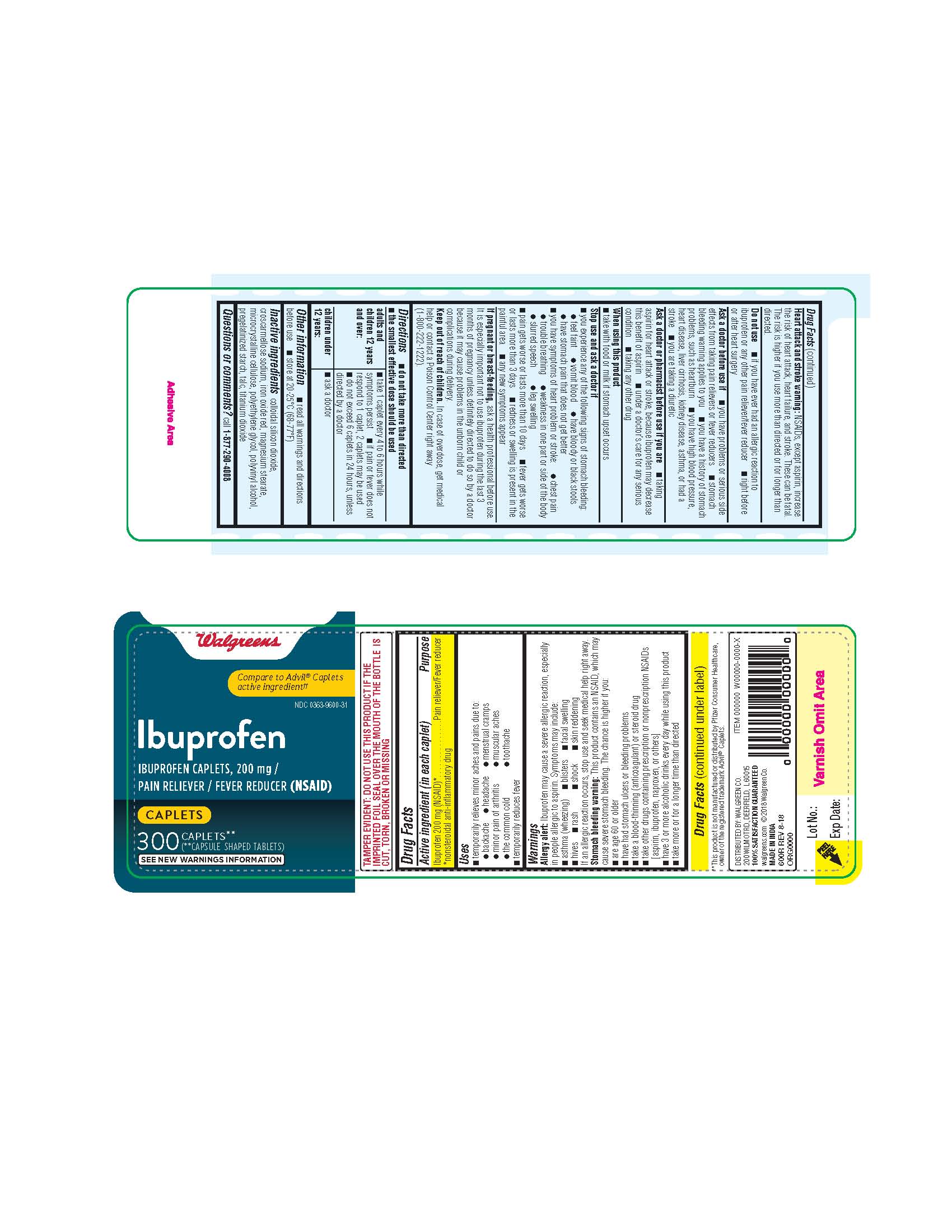
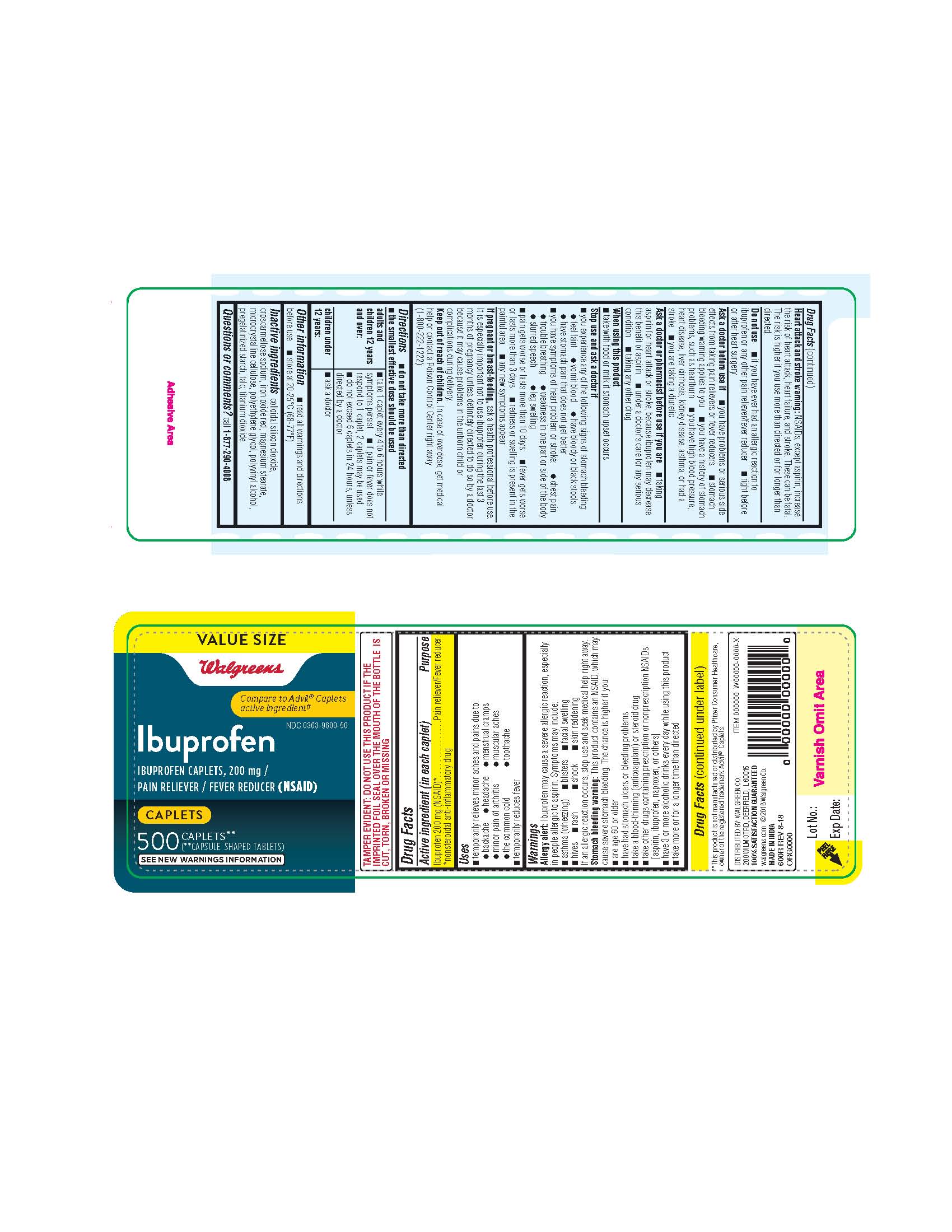
- ibuprofen 200 mg Caplets -600R INACTIVE INGREDIENTS
-
DOSAGE & ADMINISTRATION
DO NOT TAKE MORE THAN DIRECTED
THE SMALLEST EFFECTIVE DOSE SHOULD BE USED
Adults and children 12 years and over:
- take 1 caplet every 4 to 6 hours while symptom persist
- If pain does not respond to 1 caplet, 2 caplets may be used
- do not exceed 6 caplets in 24 hours, unless directed by a doctor
Children under 12 years:
- ask a doctor
- INDICATIONS & USAGE
- PURPOSE
-
WARNINGS
Allergy alert: Ibuprofen may cause a severe allergy reaction, especially in people allergic to aspirin.
Symptoms may include:asthma (wheezing)
blisters
facial swelling
hives
rash
shock
skin reddeningIf an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a NSAID, which may cause severe stomach bleeding. The chance is higher if you:are age 60 or older
have bad stomach ulcers or bleeding problems
take a blood thinning (anticoagulant) or steroid drug
take other drugs containing prescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
have 3 or more alcoholic drinks every day while using this product
the more or for a longer time than directedHeart attack and stroke warning: NSAID's except aspirin increases the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or longer than directed.
- KEEP OUT OF REACH OF CHILDREN
- Ibuprofen Caplets 200 mg 600R - 24 count
- ibuprofen 200 mg caplet 600R 50 COUNT
- Ibuprofen 200 MG CAPLETS 600R 100 COUNT
- ibuprofen 200 mg caplets 600R 300 count label
- ibuprofen 200 mg caplets 600R 500 COUNT
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0363-9600 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape CAPSULE Size 15mm Flavor Imprint Code 117 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0363-9600-42 24 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/11/2018 2 NDC: 0363-9600-05 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/11/2018 3 NDC: 0363-9600-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/11/2018 4 NDC: 0363-9600-31 300 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/11/2018 5 NDC: 0363-9600-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/11/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091239 12/11/2018 Labeler - WALGREENS (008965063) Registrant - TIME CAP LABORATORIES, INC (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(0363-9600)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.