018 Skintegrity Antibacterial Hand Soap (amber)
Skintegrity Antibacterial Hand by
Drug Labeling and Warnings
Skintegrity Antibacterial Hand by is a Otc medication manufactured, distributed, or labeled by Medline Industries, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SKINTEGRITY ANTIBACTERIAL HAND AMBER- triclosan soap
Medline Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
018 Skintegrity Antibacterial Hand Soap (amber)
Warnings
For external use only.
When using this product
- avoid contact with eyes. In case of eye contact, immediately flush with water.
Inactive ingredients
Water, Ammonium Lauryl Sulfate, Sodium Laureth Sulfate, Sodium Chloride, Cocamide DEA, Disodium Cocamido MIPA-Sulfosuccinate, Glycerin, Polyquaternium-7, DMDM Hydantoin, BHT, Fragrance, Citric Acid, Tetrasodium EDTA, Glutamic Acid, Leucine, Serine, Phenylalanine, Proline, Glycine, Valine, Isoleucine, Arginine, Citric Acid, Yellow 5, Red 33.
Package/Label Principal Display Panel
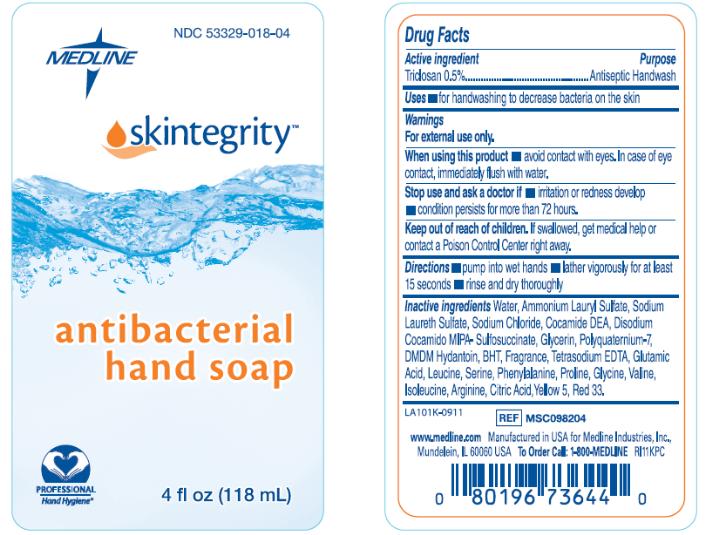
MEDLINE
NDC: 53329-018-04
skintegrity
antibacterial hand soap
4 fl oz (118 mL)
| SKINTEGRITY ANTIBACTERIAL HAND
AMBER
triclosan soap |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Medline Industries, Inc. (025460908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KUTOL PRODUCTS COMPANY | 004236139 | manufacture(53329-018) | |