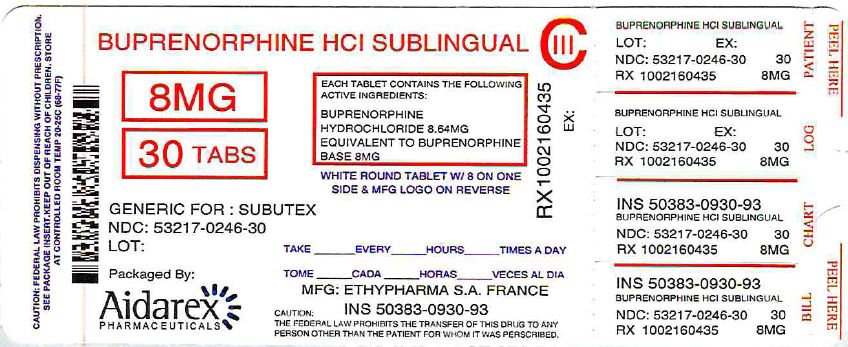
BUPRENORPHINE HYDROCHLORIDE tablet
buprenorphine hydrochloride by
Drug Labeling and Warnings
buprenorphine hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Aidarex Pharmaceuticals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BUPRENORPHINE SUBLINGUAL TABLETS safely and effectively. See full prescribing information for BUPRENORPHINE SUBLINGUAL TABLETS.
BUPRENORPHINE Sublingual Tablets, for sublingual
administration CIII
Initial U.S. Approval: 2002
RECENT MAJOR CHANGES
Warnings and Precautions Neonatal Opioid Withdrawal Syndrome (5.5) 11/2016 Adrenal Insufficiency 11/2016 INDICATIONS AND USAGE
Buprenorphine Sublingual Tablets is indicated for the treatment of opioid dependence and is preferred for induction. Prescription use of this product is limited under the Drug Addiction Treatment Act. (1)
DOSAGE AND ADMINISTRATION
Administer Buprenorphine Sublingual Tablets sublingually as a single daily dose. (2)
To avoid precipitating withdrawal, induction with Buprenorphine Sublingual Tablets should be undertaken when objective and clear signs of withdrawal are evident. (2.1) Buprenorphine and naloxone sublingual film CIII or buprenorphine and naloxone sublingual tablet CIII is generally initiated after two days of Buprenorphine Sublingual Tablets titration. (2)
DOSAGE FORMS AND STRENGTHS
Sublingual tablet: 2 mg buprenorphine and 8 mg buprenorphine. (3)
CONTRAINDICATIONS
Hypersensitivity to buprenorphine. (4)
WARNINGS AND PRECAUTIONS
- Buprenorphine can be abused in a similar manner to other
opioids.
Clinical monitoring appropriate to the patient's level of stability is essential. Multiple refills should not be prescribed early in treatment or without appropriate patient follow-up visits. (5.1) - Significant respiratory depression and death have occurred in association with buprenorphine, particularly when taken by the intravenous (IV) route in combination with benzodiazepines or other CNS depressants (including alcohol). (5.2)
- Consider dose reduction of CNS depressants, Buprenorphine Sublingual Tablets, or both in situations of concomitant prescription. (5.3)
- Store Buprenorphine Sublingual Tablets safely out of the sight and reach of children. Buprenorphine can cause severe, possibly fatal, respiratory depression in children. (5.4)
- Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy (5.5)
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off the opioid. (5.6)
- Chronic administration produces opioid-type physical
dependence.
Abrupt discontinuation or rapid dose taper may result in opioid withdrawal syndrome. (5.7) - Monitor liver function tests prior to initiation and during treatment and evaluate suspected hepatic events. (5.8)
- Do not administer Buprenorphine Sublingual Tablets to patients with known hypersensitivity to buprenorphine. (5.9)
- Buprenorphine Sublingual Tablets may precipitate opioid withdrawal signs and symptoms in individuals physically dependent on full opioid agonists if administered sublingually or parenterally before the agonist effects of other opioids have subsided. (5.10)
- Buprenorphine Sublingual Tablets are NOT appropriate as an analgesic. There have been reported deaths of opioid naïve individuals who received a 2 mg sublingual dose of buprenorphine. (5.11)
- Buprenorphine Sublingual Tablets should be used with caution in patients with moderate to severe hepatic impairment and a dose adjustment is recommended for patients with severe hepatic impairment. (5.12)
- Caution patients about the risk of driving or operating hazardous machinery. (5.13)
ADVERSE REACTIONS
Adverse events most commonly observed during clinical trials and post-marketing experience for Buprenorphine Sublingual Tablets are headache, nausea, vomiting, hyperhidrosis, constipation, signs and symptoms of withdrawal, insomnia, and pain. (6.1 and 6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-888-775-1770 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Monitor patients starting or ending CYP3A4 inhibitors or inducers for potential over or under dosing. (7.1)
- Use caution in prescribing Buprenorphine Sublingual Tablets for patients receiving benzodiazepines or other CNS depressants and warn patients against concomitant self-administration/misuse. (7.3)
- Serotonergic Drugs: Concomitant use may result in serotonin syndrome. Discontinue Buprenorphine Sublingual Tablets if serotonin syndrome is suspected. (7.4).
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Nursing mothers: Caution should be exercised when administered to a nursing woman. (8.3)
- Safety and effectiveness of Buprenorphine Sublingual Tablets in patients below the age of 16 have not been established. (8.4)
- Administer Buprenorphine Sublingual Tablets with caution to elderly or debilitated patients. (8.5)
- Buprenorphine Sublingual Tablets should be used with caution in patients with moderate to severe hepatic impairment and a dose adjustment is recommended for patients with severe hepatic impairment. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2016
- Buprenorphine can be abused in a similar manner to other
opioids.
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Induction
2.2 Maintenance
2.3 Method of Administration
2.4 Clinical Supervision
2.5 Patients With Hepatic Impairment
2.6 Unstable Patients
2.7 Stopping Treatment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Abuse Potential
5.2 Respiratory Depression
5.3 CNS Depression
5.4 Unintentional Pediatric Exposure
5.5 Neonatal Opioid Withdrawal Syndrome
5.6 Adrenal Insufficiency
5.7 Dependence
5.8 Hepatitis, Hepatic Events
5.9 Allergic Reactions
5.10 Precipitation of Opioid Withdrawal Signs and Symptoms
5.11 Use in Opioid Naïve Patients
5.12 Use in Patients With Impaired Hepatic Function
5.13 Impairment of Ability to Drive or Operate Machinery
5.14 Orthostatic Hypotension
5.15 Elevation of Cerebrospinal Fluid Pressure
5.16 Elevation of Intracholedochal Pressure
5.17 Effects in Acute Abdominal Conditions
5.18 General Precautions
6 ADVERSE REACTIONS
6.1 Adverse Events in Clinical Trials
6.2 Adverse Events - Postmarketing Experience with Buprenorphine Sublingual Tablets
7 DRUG INTERACTIONS
7.1 Cytochrome P-450 3A4 (CYP3A4) Inhibitors and Inducers
7.2 Antiretrovirals
7.3 Benzodiazepines
7.4 Serotonergic Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTIONS
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Buprenorphine Sublingual Tablets is indicated for the treatment of opioid dependence and is preferred for induction. Buprenorphine Sublingual Tablets should be used as part of a complete treatment plan to include counseling and psychosocial support.
Under the Drug Addiction Treatment Act (DATA) codified at 21 U.S.C. 823(g), prescription use of this product in the treatment of opioid dependence is limited to physicians who meet certain qualifying requirements, and who have notified the Secretary of Health and Human Services (HHS) of their intent to prescribe this product for the treatment of opioid dependence and have been assigned a unique identification number that must be included on every prescription.
-
2 DOSAGE AND ADMINISTRATION
Buprenorphine Sublingual Tablets is administered sublingually as a single daily dose. Buprenorphine Sublingual Tablets contain no naloxone HCL and is preferred for use only during induction. Following induction, Buprenorphine and Naloxone Sublingual Film or Buprenorphine and Naloxone Sublingual Tablets is preferred due to the presence of naloxone when clinical use includes unsupervised administration. The use of Buprenorphine Sublingual Tablets for unsupervised administration should be limited to those patients who cannot tolerate Buprenorphine and Naloxone Sublingual Film or Buprenorphine and Naloxone Sublingual Tablets; for example, those patients who have been shown to be hypersensitive to naloxone.
Medication should be prescribed in consideration of the frequency of visits. Provision of multiple refills is not advised early in treatment or without appropriate patient follow-up visits.
2.1 Induction
Prior to induction, consideration should be given to the type of opioid dependence (i.e. long- or short-acting opioid), the time since last opioid use, and the degree or level of opioid dependence. To avoid precipitating withdrawal, induction with Buprenorphine Sublingual Tablets should be undertaken when objective and clear signs of withdrawal are evident.
It is recommended that an adequate treatment dose, titrated to clinical effectiveness, should be achieved as rapidly as possible. In a one-month study, patients received 8 mg of Buprenorphine Sublingual Tablets on Day 1 and 16 mg Buprenorphine Sublingual Tablets on Day 2. From Day 3 onward, patients received either buprenorphine and naloxone sublingual tablet or Buprenorphine Sublingual Tablets at the same buprenorphine dose as Day 2 based on their assigned treatment. Induction in the studies of buprenorphine solution was accomplished over 3 to 4 days, depending on the target dose. In some studies, gradual induction over several days led to a high rate of drop-out of buprenorphine patients during the induction period.
Patients taking heroin or other short-acting opioids: At treatment initiation, the dose of Buprenorphine Sublingual Tablets should be administered at least 4 hours after the patient last used opioids or preferably when moderate objective signs of opioid withdrawal appear.
Patients on methadone or other long-acting opioids: There is little controlled experience with the transfer of methadone-maintained patients to buprenorphine. Available evidence suggests that withdrawal signs and symptoms are possible during induction onto buprenorphine. Withdrawal appears more likely in patients maintained on higher doses of methadone (greater than 30 mg) and when the first buprenorphine dose is administered shortly after the last methadone dose. Buprenorphine Sublingual Tablets dosing should be initiated preferably when moderate objective signs of opioid withdrawal appear.
2.2 Maintenance
- Buprenorphine and naloxone is preferred for maintenance treatment.
- Where buprenorphine is used in maintenance in patients who cannot tolerate the presence of naloxone, the dosage of buprenorphine should be progressively adjusted in increments / decrements of 2 mg or 4 mg buprenorphine to a level that holds the patient in treatment and suppresses opioid withdrawal signs and symptoms.
- The maintenance dose is generally in the range of 4 mg to
24 mg buprenorphine per day depending on the individual
patient.
Doses higher than this have not been demonstrated to provide any clinical advantage.
2.3 Method of Administration
Buprenorphine Sublingual Tablets should be placed under the tongue until it is dissolved. For doses requiring the use of more than two tablets, patients are advised to either place all the tablets at once or alternatively (if they cannot fit in more than two tablets comfortably), place two tablets at a time under the tongue. Either way, the patients should continue to hold the tablets under the tongue until they dissolve; swallowing the tablets reduces the bioavailability of the drug. To ensure consistency in bioavailability, patients should follow the same manner of dosing with continued use of the product.
Proper administration technique should be demonstrated to the patient.
2.4 Clinical Supervision
Treatment should be initiated with supervised administration, progressing to unsupervised administration as the patient's clinical stability permits. The use of buprenorphine for unsupervised administration should be limited to those patients who cannot tolerate buprenorphine and naloxone, for example those patients with known hypersensitivity to naloxone. Buprenorphine and naloxone and buprenorphine are both subject to diversion and abuse. When determining the size of the prescription quantity for unsupervised administration, consider the patient's level of stability, the security of his or her home situation, and other factors likely to affect the ability of the patient to manage supplies of take-home medication.
Ideally, patients should be seen at reasonable intervals (e.g., at least weekly during the first month of treatment) based upon the individual circumstances of the patient. Medication should be prescribed in consideration of the frequency of visits. Provision of multiple refills is not advised early in treatment or without appropriate patient follow-up visits. Periodic assessment is necessary to determine compliance with the dosing regimen, effectiveness of the treatment plan, and overall patient progress.
Once a stable dosage has been achieved and patient assessment (e.g., urine drug screening) does not indicate illicit drug use, less frequent follow-up visits may be appropriate. A once-monthly visit schedule may be reasonable for patients on a stable dosage of medication who are making progress toward their treatment objectives. Continuation or modification of pharmacotherapy should be based on the physician's evaluation of treatment outcomes and objectives such as:
- Absence of medication toxicity.
- Absence of medical or behavioral adverse effects.
- Responsible handling of medications by the patient.
- Patient's compliance with all elements of the treatment plan (including recovery-oriented activities, psychotherapy, and/or other psychosocial modalities).
- Abstinence from illicit drug use (including problematic alcohol and/or benzodiazepine use).
If treatment goals are not being achieved, the physician should re-evaluate the appropriateness of continuing the current treatment.
2.5 Patients With Hepatic Impairment
Severe hepatic impairment: Consider reducing the starting and titration incremental dose by half compared to patients with normal liver function, and monitor for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine.
Moderate hepatic impairment: Although no dose adjustment is necessary for patients with moderate hepatic impairment, Buprenorphine Sublingual Tablets should be used with caution in these patients and prescribers should monitor patients for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine.
Mild hepatic impairment: No clinically significant differences in pharmacokinetic parameters were observed in subjects with mild hepatic impairment. No dose adjustment is needed in patients with mild hepatic impairment. [see Warnings and Precautions (5.11)].
2.6 Unstable Patients
Physicians will need to decide when they cannot appropriately provide further management for particular patients. For example, some patients may be abusing or dependent on various drugs, or unresponsive to psychosocial intervention such that the physician does not feel that he/she has the expertise to manage the patient. In such cases, the physician may want to assess whether to refer the patient to a specialist or more intensive behavioral treatment environment. Decisions should be based on a treatment plan established and agreed upon with the patient at the beginning of treatment.
Patients who continue to misuse, abuse, or divert buprenorphine products or other opioids should be provided with, or referred to, more intensive and structured treatment.
2.7 Stopping Treatment
The decision to discontinue therapy with buprenorphine and naloxone or buprenorphine after a period of maintenance should be made as part of a comprehensive treatment plan. Both gradual and abrupt discontinuation of buprenorphine has been used, but the data are insufficient to determine the best method of dose taper at the end of treatment.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Buprenorphine Sublingual Tablets should not be administered to patients who have been shown to be hypersensitive to buprenorphine, as serious adverse reactions, including anaphylactic shock, have been reported. [see Warnings and Precautions (5.8)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Abuse Potential
Buprenorphine can be abused in a manner similar to other opioids, legal or illicit. Prescribe and dispense buprenorphine with appropriate precautions to minimize risk of misuse, abuse, or diversion, and ensure appropriate protection from theft, including in the home. Clinical monitoring appropriate to the patient's level of stability is essential. Multiple refills should not be prescribed early in treatment or without appropriate patient follow-up visits. [see Drug Abuse and Dependence (9.2)].
5.2 Respiratory Depression
Buprenorphine, particularly when taken by the IV route, in combination with benzodiazepines or other CNS depressants (including alcohol), has been associated with significant respiratory depression and death. Many, but not all post-marketing reports regarding coma and death associated with the concomitant use of buprenorphine and benzodiazepines involved misuse by self-injection. Deaths have also been reported in association with concomitant administration of buprenorphine with other depressants such as alcohol or other CNS depressant drugs. Patients should be warned of the potential danger of self-administration of benzodiazepines or other depressants while under treatment with Buprenorphine Sublingual Tablets. [see Drug Interactions (7.3)].
In the case of overdose, the primary management should be the re-establishment of adequate ventilation with mechanical assistance of respiration, if required. Naloxone may be of value for the management of buprenorphine overdose. Higher than normal doses and repeated administration may be necessary.
Buprenorphine Sublingual Tablets should be used with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression).
5.3 CNS Depression
Patients receiving buprenorphine in the presence of opioid analgesics, general anesthetics, benzodiazepines, phenothiazines, other tranquilizers, sedative/hypnotics or other CNS depressants (including alcohol) may exhibit increased CNS depression. Consider dose reduction of CNS depressants, Buprenorphine Sublingual Tablets, or both in situations of concomitant prescription. [see Drug Interactions (7.3)].
5.4 Unintentional Pediatric Exposure
Buprenorphine can cause severe, possibly fatal, respiratory depression in children who are accidentally exposed to it. Store buprenorphine-containing medications safely out of the sight and reach of children and destroy any unused medication appropriately. [see Patient Counseling (17)].
5.5 Neonatal Opioid Withdrawal Syndrome
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy, whether that use is medically-authorized or illicit. Unlike opioid withdrawal syndrome in adults, NOWS may be life-threatening if not recognized and treated in the neonate. Healthcare professionals should observe newborns for signs of NOWS and manage accordingly [see Use in Specific Populations (8.1)].
Advise pregnant women receiving opioid addiction treatment with Buprenorphine Sublingual Tablets of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations (8.1)]. This risk must be balanced against the risk of untreated opioid addiction which often results in continued or relapsing illicit opioid use and is associated with poor pregnancy outcomes. Therefore, prescribers should discuss the importance and benefits of management of opioid addiction throughout pregnancy.
5.6 Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
5.7 Dependence
Buprenorphine is a partial agonist at the mu-opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by withdrawal signs and symptoms upon abrupt discontinuation or rapid taper. The withdrawal syndrome is typically milder than seen with full agonists and may be delayed in onset. Buprenorphine can be abused in a manner similar to other opioids. This should be considered when prescribing or dispensing buprenorphine in situations when the clinician is concerned about an increased risk of misuse, abuse, or diversion. [see Drug Abuse and Dependence (9.3)].
5.8 Hepatitis, Hepatic Events
Cases of cytolytic hepatitis and hepatitis with jaundice have been observed in individuals receiving buprenorphine in clinical trials and through post-marketing adverse event reports. The spectrum of abnormalities ranges from transient asymptomatic elevations in hepatic transaminases to case reports of death, hepatic failure, hepatic necrosis, hepatorenal syndrome, and hepatic encephalopathy. In many cases, the presence of pre-existing liver enzyme abnormalities, infection with hepatitis B or hepatitis C virus, concomitant usage of other potentially hepatotoxic drugs, and ongoing injecting drug use may have played a causative or contributory role. In other cases, insufficient data were available to determine the etiology of the abnormality. Withdrawal of buprenorphine has resulted in amelioration of acute hepatitis in some cases; however, in other cases no dose reduction was necessary. The possibility exists that buprenorphine had a causative or contributory role in the development of the hepatic abnormality in some cases. Liver function tests, prior to initiation of treatment is recommended to establish a baseline. Periodic monitoring of liver function during treatment is also recommended. A biological and etiological evaluation is recommended when a hepatic event is suspected. Depending on the case, Buprenorphine Sublingual Tablets may need to be carefully discontinued to prevent withdrawal signs and symptoms and a return by the patient to illicit drug use, and strict monitoring of the patient should be initiated.
5.9 Allergic Reactions
Cases of hypersensitivity to buprenorphine products have been reported both in clinical trials and in the post-marketing experience. Cases of bronchospasm, angioneutrotic edema, and anaphylactic shock have been reported. The most common signs and symptoms include rashes, hives, and pruritus. A history of hypersensitivity to buprenorphine is a contraindication to the use of Buprenorphine Sublingual Tablets.
5.10 Precipitation of Opioid Withdrawal Signs and Symptoms
Because of the partial agonist properties of buprenorphine, Buprenorphine Sublingual Tablets may precipitate opioid withdrawal signs and symptoms in individuals physically dependent on full opioid agonists if administered sublingually or parenterally before the agonist effects of other opioids have subsided.
5.11 Use in Opioid Naïve Patients
There have been reported deaths of opioid naïve individuals who received a 2 mg dose of buprenorphine as a sublingual tablet for analgesia. Buprenorphine Sublingual Tablets are not appropriate as an analgesic.
5.12 Use in Patients With Impaired Hepatic Function
In a pharmacokinetic study, buprenorphine plasma levels were found to be higher and the half-life was found to be longer in subjects with moderate and severe hepatic impairment, but not in subjects with mild hepatic impairment.
For patients with severe hepatic impairment, a dose adjustment is recommended, and patients with moderate or severe hepatic impairment should be monitored for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)].
5.13 Impairment of Ability to Drive or Operate Machinery
Buprenorphine Sublingual Tablets may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery, especially during treatment induction and dose adjustment. Patients should be cautioned about driving or operating hazardous machinery until they are reasonably certain that buprenorphine therapy does not adversely affect his or her ability to engage in such activities.
5.14 Orthostatic Hypotension
Like other opioids, Buprenorphine Sublingual Tablets may produce orthostatic hypotension in ambulatory patients.
5.15 Elevation of Cerebrospinal Fluid Pressure
Buprenorphine, like other opioids, may elevate cerebrospinal fluid pressure and should be used with caution in patients with head injury, intracranial lesions and other circumstances when cerebrospinal pressure may be increased. Buprenorphine can produce miosis and changes in the level of consciousness that may interfere with patient evaluation.
5.16 Elevation of Intracholedochal Pressure
Buprenorphine has been shown to increase intracholedochal pressure, as do other opioids, and thus should be administered with caution to patients with dysfunction of the biliary tract.
5.17 Effects in Acute Abdominal Conditions
As with other opioids, buprenorphine may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
5.18 General Precautions
Buprenorphine Sublingual Tablets should be administered with caution in debilitated patients and those with myxedema or hypothyroidism; adrenal cortical insufficiency (e.g., Addison's disease); CNS depression or coma; toxic psychoses; prostatic hypertrophy or urethral stricture; acute alcoholism; delirium tremens; or kyphoscoliosis.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Adverse Events in Clinical Trials
The safety of Buprenorphine Sublingual Tablets was supported by clinical trials using Buprenorphine Sublingual Tablets, buprenorphine and naloxone sublingual tablets and other trials using buprenorphine sublingual solutions. In total, safety data were available from 3214 opioid-dependent subjects exposed to buprenorphine at doses in the range used in treatment of opioid addiction.
Few differences in adverse event profile were noted between Buprenorphine Sublingual Tablets or buprenorphine administered as a sublingual solution.
The following adverse events were reported to occur by at least 5% of patients in a 4-week study (Table 1).
Table 1: Adverse Events ≥5% by Body System and Treatment Group in a 4-week Study N(%) N(%) Body System /Adverse Event (COSTART Terminology) Buprenorphine
Sublingual Tablets
16 mg/day
N=103Placebo
N=107Body as a Whole Asthenia 5 (4.9%) 7 (6.5%) Chills 8 (7.8%) 8 (7.5%) Headache 30 (29.1%) 24 (22.4%) Infection 12 (11.7%) 7 (6.5%) Pain 19 (18.4%) 20 (18.7%) Pain Abdomen 12 (11.7%) 7 (6.5%) Pain Back 8 (7.8%) 12 (11.2%) Withdrawal Syndrome 19 (18.4%) 40 (37.4%) Cardiovascular System Vasodilation 4 (3.9%) 7 (6.5%) Digestive System Constipation 8 (7.8%) 3 (2.8%) Diarrhea 5 (4.9%) 16 (15.0%) Nausea 14 (13.6%) 12 (11.2%) Vomiting 8 (7.8%) 5 (4.7%) Nervous System Insomnia 22 (21.4%) 17 (15.9%) Respiratory System Rhinitis 10 (9.7%) 14 (13.1%) Skin and Appendages Sweating 13 (12.6%) 11 (10.3%) The adverse event profile of buprenorphine was also characterized in the dose-controlled study of buprenorphine solution, over a range of doses in four months of treatment. Table 2 shows adverse events reported by at least 5% of subjects in any dose group in the dose-controlled study.
Table 2: Adverse Events (≥5%) by Body System and Treatment Group in a 16-week Study - * Sublingual solution. Doses in this table cannot necessarily be delivered in tablet form, but for comparison purposes:
"Very low" dose (1 mg solution) would be less than a tablet dose of 2 mg.
"Low" dose (4 mg solution) approximates a 6 mg tablet dose.
"Moderate" dose (8 mg solution) approximates a 12 mg tablet dose.
"High" dose (16 mg solution) approximates a 24 mg tablet dose.Body System/
Adverse Event (COSTART Terminology)Buprenorphine Dose* Very Low*
(N=184)Low*
(N=180)Moderate*
(N=186)High*
(N=181)Total*
(N=731)N (%) N (%) N (%) N (%) N (%) Body as a Whole Abscess 9 (5%) 2 (1%) 3 (2%) 2 (1%) 16 (2%) Asthenia 26 (14%) 28 (16%) 26 (14%) 24 (13%) 104 (14%) Chills 11 (6%) 12 (7%) 9 (5%) 10 (6%) 42 (6%) Fever 7 (4%) 2 (1%) 2 (1%) 10 (6%) 21 (3%) Flu Syndrome 4 (2%) 13 (7%) 19 (10%) 8 (4%) 44 (6%) Headache 51 (28%) 62 (34%) 54 (29%) 53 (29%) 220 (30%) Infection 32 (17%) 39 (22%) 38 (20%) 40 (22%) 149 (20%) Injury Accidental 5 (3%) 10 (6%) 5 (3%) 5 (3%) 25 (3%) Pain 47 (26%) 37 (21%) 49 (26%) 44 (24%) 177 (24%) Pain Back 18 (10%) 29 (16%) 28 (15%) 27 (15%) 102 (14%) Withdrawal Syndrome 45 (24%) 40 (22%) 41 (22%) 36 (20%) 162 (22%) Digestive System Constipation 10 (5%) 23 (13%) 23 (12%) 26 (14%) 82 (11%) Diarrhea 19 (10%) 8 (4%) 9 (5%) 4 (2%) 40 (5%) Dyspepsia 6 (3%) 10 (6%) 4 (2%) 4 (2%) 24 (3%) Nausea 12 (7%) 22 (12%) 23 (12%) 18 (10%) 75 (10%) Vomiting 8 (4%) 6 (3%) 10 (5%) 14 (8%) 38 (5%) Nervous System Anxiety 22 (12%) 24 (13%) 20 (11%) 25 (14%) 91 (12%) Depression 24 (13%) 16 (9%) 25 (13%) 18 (10%) 83 (11%) Dizziness 4 (2%) 9 (5%) 7 (4%) 11 (6%) 31 (4%) Insomnia 42 (23%) 50 (28%) 43 (23%) 51 (28%) 186 (25%) Nervousness 12 (7%) 11 (6%) 10 (5%) 13 (7%) 46 (6%) Somnolence 5 (3%) 13 (7%) 9 (5%) 11 (6%) 38 (5%) Respiratory System Cough Increase 5 (3%) 11 (6%) 6 (3%) 4 (2%) 26 (4%) Pharyngitis 6 (3%) 7 (4%) 6 (3%) 9 (5%) 28 (4%) Rhinitis 27 (15%) 16 (9%) 15 (8%) 21 (12%) 79 (11%) Skin and Appendages Sweat 23 (13%) 21 (12%) 20 (11%) 23 (13%) 87 (12%) Special Senses Runny Eyes 13 (7%) 9 (5%) 6 (3%) 6 (3%) 34 (5%) 6.2 Adverse Events - Postmarketing Experience with Buprenorphine Sublingual Tablets
The most frequently reported postmarketing adverse events with buprenorphine not observed in clinical trials, excluding drug exposure during pregnancy, was drug misuse or abuse.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in Buprenorphine Sublingual Tablets.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
- * Sublingual solution. Doses in this table cannot necessarily be delivered in tablet form, but for comparison purposes:
-
7 DRUG INTERACTIONS
7.1 Cytochrome P-450 3A4 (CYP3A4) Inhibitors and Inducers
Buprenorphine is metabolized to norbuprenorphine primarily by cytochrome CYP3A4; therefore, potential interactions may occur when Buprenorphine Sublingual Tablets is given concurrently with agents that affect CYP3A4 activity. The concomitant use of Buprenorphine Sublingual Tablets with CYP3A4 inhibitors (e.g., azole antifungals such as ketoconazole, macrolide antibiotics such as erythromycin, and HIV protease inhibitors) should be monitored and may require dose-reduction of one or both agents.
The interaction of buprenorphine with many CYP3A4 inducers has not been studied; therefore, it is recommended that patients receiving Buprenorphine Sublingual Tablets be monitored for signs and symptoms of opioid withdrawal if inducers of CYP3A4 (e.g., phenobarbital, carbamazepine, phenytoin, rifampicin) are co-administered. [see Clinical Pharmacology (12.3)].
7.2 Antiretrovirals
Three classes of antiretroviral agents have been evaluated for CYP3A4 interactions with buprenorphine. Nucleoside reverse transcriptase inhibitors (NRTIs) do not appear to induce or inhibit the P450 enzyme pathway, thus no interactions with buprenorphine are expected. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are metabolized principally by CYP3A4. Efavirenz, nevirapine and etravirine are known CYP3A inducers whereas delaviridine is a CYP3A inhibitor. Significant pharmacokinetic interactions between NNRTIs (e.g., efavirenz and delavirdine) and buprenorphine have been shown in clinical studies, but these pharmacokinetic interactions did not result in any significant pharmacodynamic effects. It is recommended that patients who are on chronic buprenorphine treatment have their dose monitored if NNRTIs are added to their treatment regimen. Studies have shown some antiretroviral protease inhibitors (PIs) with CYP3A4 inhibitory activity (nelfinavir, lopinavir/ritonavir, ritonavir) have little effect on buprenorphine pharmacokinetic and no significant pharmacodynamic effects. Other PIs with CYP3A4 inhibitory activity (atazanavir and atazanavir/ritonavir) resulted in elevated levels of buprenorphine and norbuprenorphine and patients in one study reported increased sedation. Symptoms of opioid excess have been found in post-marketing reports of patients receiving buprenorphine and atazanavir with and without ritonavir concomitantly. Monitoring of patients taking buprenorphine and atazanavir with and without ritonavir is recommended, and dose reduction of buprenorphine may be warranted.
7.3 Benzodiazepines
There have been a number of post-marketing reports regarding coma and death associated with the concomitant use of buprenorphine and benzodiazepines. In many, but not all, of these cases, buprenorphine was misused by self-injection. Preclinical studies have shown that the combination of benzodiazepines and buprenorphine altered the usual ceiling effect on buprenorphine-induced respiratory depression, making the respiratory effects of buprenorphine appear similar to those of full opioid agonists. Buprenorphine Sublingual Tablets should be prescribed with caution to patients taking benzodiazepines or other drugs that act on the CNS, regardless of whether these drugs are taken on the advice of a physician or are being abused/misused. Patients should be warned that it is extremely dangerous to self-administer non-prescribed benzodiazepines while taking Buprenorphine Sublingual Tablets, and should also be cautioned to use benzodiazepines concurrently with Buprenorphine Sublingual Tablets only as directed by their physician.
7.4 Serotonergic Drugs
The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system, such as selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that effect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), and monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue), has resulted in serotonin syndrome.
If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue Buprenorphine Sublingual Tablets if serotonin syndrome is suspected.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Risk Summary
There are no adequate and well-controlled studies of Buprenorphine Sublingual Tablets in pregnant women. Limited published data on use of buprenorphine, the active ingredient in Buprenorphine Sublingual Tablets, in pregnancy, have not shown an increased risk of major malformations. All pregnancies, regardless of drug exposure, have a background risk of 2% to 4% for major birth defects, and 15% to 20% for pregnancy loss. Reproductive and developmental studies in rats and rabbits identified adverse events at clinically relevant doses. Pre-and postnatal development studies in rats demonstrated dystocia, increased neonatal deaths, and developmental delays. No clear teratogenic effects were seen with a range of doses equivalent to or greater than the human dose. However, in a few studies, some events such as acephalus, omphalocele, and skeletal abnormalities were observed but these findings were not clearly treatment-related. Embryofetal death was also observed in both rats and rabbits.Buprenorphine Sublingual Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Disease-associated maternal and embryo-fetal risk
Untreated opioid addiction in pregnancy is associated with adverse obstetrical outcomes such as low birth weight, preterm birth, and fetal death. In addition, untreated opioid addiction often results in continued or relapsing illicit opioid use.Fetal/neonatal adverse reactions
Neonatal opioid withdrawal syndrome may occur in newborn infants of mothers who are receiving treatment with Buprenorphine Sublingual Tablets.Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea, and/or failure to gain weight. Signs of neonatal withdrawal usually occur in the first days after birth. The duration and severity of neonatal opioid withdrawal syndrome may vary. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. [see Warnings and Precautions (5.5)].
Labor or Delivery
As with all opioids, use of buprenorphine prior to delivery may result in respiratory depression in the newborn.Closely monitor neonates for signs of respiratory depression. An opioid antagonist such as naloxone should be available for reversal of opioid induced respiratory depression in the neonate.
Data
Human Data
Studies have been conducted to evaluate neonatal outcomes in women exposed to buprenorphine during pregnancy. Limited published data on malformations from trials, observational studies, case series, and case reports on buprenorphine use in pregnancy have not shown an increased risk of major malformations. Based on these studies the incidence of neonatal abstinence syndrome is not clear and there does not appear to be a dose-response relationship.Animal Data
Buprenorphine was not teratogenic in rats or rabbits after IM or subcutaneous (SC) doses up to 5 mg/kg/day (estimated exposure was approximately 3 and 6 times, respectively, the recommended human daily sublingual dose of 16 mg on a mg/m2 basis), after IV doses up to 0.8 mg/kg/day (estimated exposure was approximately 0.5 times and equal to, respectively, the recommended human daily sublingual dose of 16 mg on a mg/m2 basis), or after oral doses up to 160 mg/kg/day in rats (estimated exposure was approximately 95 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis) and 25 mg/kg/day in rabbits (estimated exposure was approximately 30 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis). Significant increases in skeletal abnormalities (e.g., extra thoracic vertebra or thoraco-lumbar ribs) were noted in rats after SC administration of 1 mg/kg/day and up (estimated exposure was approximately 0.6 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis), but were not observed at oral doses up to 160 mg/kg/day. Increases in skeletal abnormalities in rabbits after IM administration of 5 mg/kg/day (estimated exposure was approximately 6 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis) or oral administration of 1 mg/kg/day or greater (estimated exposure was approximately equal to the recommended human daily sublingual dose of 16 mg on a mg/m2 basis) were not statistically significant.In rabbits, buprenorphine produced statistically significant pre-implantation losses at oral doses of 1 mg/kg/day or greater and post- implantation losses that were statistically significant at IV doses of 0.2 mg/kg/day or greater (estimated exposure was approximately 0.3 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis).
Dystocia was noted in pregnant rats treated intramuscularly with buprenorphine 5 mg/kg/day (approximately 3 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis). Fertility, peri- and post-natal development studies with buprenorphine in rats indicated increases in neonatal mortality after oral doses of 0.8 mg/kg/day and up (approximately 0.5 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis), after IM doses of 0.5 mg/kg/day and up (approximately 0.3 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis), and after SC doses of 0.1 mg/kg/day and up (approximately 0.06 times the recommended human daily sublingual dose of 16 mg on mg/m2 basis). An apparent lack of milk production during these studies likely contributed to the decreased pup viability and lactation indices. Delays in the occurrence of righting reflex and startle response were noted in rat pups at an oral dose of 80 mg/kg/day (approximately 50 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis).
8.3 Nursing Mothers
Risk Summary
Based on two studies in 13 lactating women, buprenorphine and its metabolite norbuprenorphine are present in low levels in human milk and infant urine, and available data have not shown adverse reactions in breastfed infants. There are no data on the combination product buprenorphine/naloxone in breastfeeding, however oral absorption of naloxone is minimal. Caution should be exercised when buprenorphine is administered to a nursing woman. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for buprenorphine and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.Clinical Considerations
Advise the nursing mother taking buprenorphine to monitor the infant for increased drowsiness and breathing difficulties.
Data
Based on limited data from a study of 6 lactating women who were taking a median oral dose of buprenorphine of 0.29 mg/kg/day 5 to 8 days after delivery, breast milk contained a median infant dose of 0.42 mcg/kg/day of buprenorphine and 0.33 mcg/kg/day of norbuprenorphine, which are equal to 0.2% and 0.12% of the maternal weight-adjusted dose.Based on limited data from a study of 7 lactating women who were taking a median oral dose of buprenorphine of 7 mg/day an average of 1.12 months after delivery, the mean milk concentrations of buprenorphine and norbuprenorphine were 3.65 mcg/L and 1.94 mcg/L respectively. Based on the limited data from this study, and assuming milk consumption of 150 mL/kg/day, an exclusively breastfed infant would receive an estimated mean of 0.55 mcg/kg/day of buprenorphine and 0.29 mcg/kg/day of norbuprenorphine, which are 0.38% and 0.18% of the maternal weight-adjusted dose.
No adverse reactions were observed in the infants in these two studies.
Females and Males of Reproductive Potential
Infertility
Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6.2)].
8.4 Pediatric Use
The safety and effectiveness of Buprenorphine Sublingual Tablet has not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of Buprenorphine Sublingual Tablets, buprenorphine and naloxone sublingual film, or buprenorphine and naloxone sublingual tablet did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
The effects of hepatic impairment on the pharmacokinetics of buprenorphine were evaluated in a pharmacokinetic study. Buprenorphine is extensively metabolized in the liver and buprenorphine plasma levels were found to be higher and the half-life was found to be longer in subjects with moderate and severe hepatic impairment, but not in subjects with mild hepatic impairment.
For patients with severe hepatic impairment, a dose adjustment is recommended, and patients with moderate or severe hepatic impairment should be monitored for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine. [see Dosage and Administration (2.5), Warnings and Precautions (5.12) and Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Buprenorphine is a Schedule III narcotic under the Controlled Substances Act.
Under the Drug Addiction Treatment Act (DATA) codified at 21 U.S.C. 823(g), prescription use of this product in the treatment of opioid dependence is limited to physicians who meet certain qualifying requirements, and who have notified the Secretary of Health and Human Services (HHS) of their intent to prescribe this product for the treatment of opioid dependence and have been assigned a unique identification number that must be included on every prescription.
9.2 Abuse
Buprenorphine, like morphine and other opioids, has the potential for being abused and is subject to criminal diversion. This should be considered when prescribing or dispensing buprenorphine in situations when the clinician is concerned about an increased risk of misuse, abuse, or diversion. Healthcare professionals should contact their state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Patients who continue to misuse, abuse, or divert, buprenorphine products or other opioids should be provided or referred for more intensive and structured treatment.
Abuse of buprenorphine poses a risk of overdose and death. This risk is increased with the abuse of buprenorphine and alcohol and other substances, especially benzodiazepines.
The physician may be able to more easily detect misuse or diversion by maintaining records of medication prescribed including date, dose, quantity, frequency of refills, and renewal requests of medication prescribed.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper handling and storage of the medication are appropriate measures that help to limit abuse of opioid drugs.
9.3 Dependence
Buprenorphine is a partial agonist at the mu-opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by moderate withdrawal signs and symptoms upon abrupt discontinuation or rapid taper. The withdrawal syndrome is typically milder than seen with full agonists and may be delayed in onset. [see Warnings and Precautions (5.5)].
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy [see Warnings and Precautions (5.5)]
-
10 OVERDOSAGE
The manifestations of acute overdose include pinpoint pupils, sedation, hypotension, respiratory depression, and death.
In the event of overdose, the respiratory and cardiac status of the patient should be monitored carefully. When respiratory or cardiac functions are depressed, primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Oxygen, IV fluids, vasopressors, and other supportive measures should be employed as indicated.
In the case of overdose, the primary management should be the re-establishment of adequate ventilation with mechanical assistance of respiration, if required. Naloxone may be of value for the management of buprenorphine overdose. Higher than normal doses and repeated administration may be necessary. The long duration of action of buprenorphine should be taken into consideration when determining the length of treatment and medical surveillance needed to reverse the effects of an overdose. Insufficient duration of monitoring may put patients at risk.
-
11 DESCRIPTIONS
Buprenorphine Sublingual Tablets are uncoated round white tablets intended for sublingual administration. The tablets contain buprenorphine HCL and are available in two dosage strengths, 2 mg buprenorphine and 8 mg buprenorphine (as free base). Each tablet also contains citric acid, cornstarch, lactose monohydrate, mannitol, povidone K30, sodium citrate anhydrous and sodium stearyl fumerate. The 2 mg buprenorphine tablet is debossed with a "2" on one side and an "→" on the other. The 8 mg buprenorphine tablet is debossed with a "8" on one side and an "→" on the other.
Chemically, buprenorphine HCl is (2S)-2-[17-Cyclopropylmethyl-4,5α-epoxy-3-hydroxy-6-methoxy-6α,14-ethano-14α- morphinan-7α-yl]-3,3dimethylbutan-2-ol hydrochloride.
It has the following chemical structure:
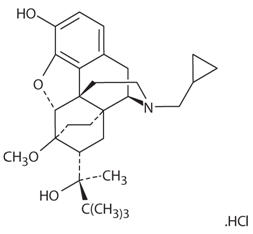
Buprenorphine HCl has the molecular formula C29H41 NO4 ∙ HCl and the molecular weight is 504.10. It is a white or off-white crystalline powder, sparingly soluble in water, freely soluble in methanol, soluble in alcohol and practically insoluble in cyclohexane.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Buprenorphine Sublingual Tablets contain buprenorphine. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor.
12.2 Pharmacodynamics
Subjective Effects: Comparisons of buprenorphine to full opioid agonists such as methadone and hydromorphone suggest that sublingual buprenorphine produces typical opioid agonist effects which are limited by a ceiling effect.
Opioid agonist ceiling-effects were also observed in a double-blind, parallel group, dose-ranging comparison of single doses of buprenorphine sublingual solution (1, 2, 4, 8, 16, or 32 mg), placebo and a full agonist control at various doses. The treatments were given in ascending dose order at intervals of at least one week to 16 opioid-experienced subjects who were not physically dependent. Both active drugs produced typical opioid agonist effects. For all measures for which the drugs produced an effect, buprenorphine produced a dose-related response. However, in each case, there was a dose that produced no further effect. In contrast, the highest dose of the full agonist control always produced the greatest effects. Agonist objective rating scores remained elevated for the higher doses of buprenorphine (8mg to 32 mg) longer than for the lower doses and did not return to baseline until 48 hours after drug administration. The onset of effects appeared more rapidly with buprenorphine than with the full agonist control, with most doses nearing peak effect after 100 minutes for buprenorphine compared to 150 minutes for the full agonist control.
Physiologic Effects: Buprenorphine in IV (2, 4, 8, 12 and 16 mg) and sublingual (12 mg) doses have been administered to opioid- experienced subjects who were not physically dependent to examine cardiovascular, respiratory and subjective effects at doses comparable to those used for treatment of opioid dependence. Compared to placebo, there were no statistically significant differences among any of the treatment conditions for blood pressure, heart rate, respiratory rate, O2 saturation, or skin temperature across time. Systolic BP was higher in the 8 mg group than placebo (3-hour AUC values). Minimum and maximum effects were similar across all treatments. Subjects remained responsive to low voice and responded to computer prompts. Some subjects showed irritability, but no other changes were observed.
The respiratory effects of sublingual buprenorphine were compared with the effects of methadone in a double-blind, parallel group, dose ranging comparison of single doses of buprenorphine sublingual solution (1, 2, 4, 8, 16, or 32 mg) and oral methadone (15, 30, 45, or 60 mg) in non-dependent, opioid-experienced volunteers. In this study, hypoventilation not requiring medical intervention was reported more frequently after buprenorphine doses of 4 mg and higher than after methadone. Both drugs decreased O2 saturation to the same degree.
Effects on the Endocrine System:
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to hormonal changes that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions (6.2)].
12.3 Pharmacokinetics
Absorption: Plasma levels of buprenorphine increased with the sublingual dose of Buprenorphine Sublingual Tablets (Table 3). There was wide inter-patient variability in the sublingual absorption of buprenorphine, but within subjects the variability was low. Both Cmax and AUC of buprenorphine increased in a linear fashion with the increase in dose (in the range of 4 to 16 mg), although the increase was not directly dose-proportional.
Table 3: Pharmacokinetic Parameters of Buprenorphine and Norbuprenorphine After the Sublingual Administration of Buprenorphine Sublingual Tablets aSource: Study Report 20-A78-AU
bSource: Study Report 20-276-SA
cSource: Study Report 20-A79-AUDose Analyte Mean
SDCmax
(ng/mL)Tmax
(h)AUCinf
(h∙ng/mL)t1/2
(h)2 mga Buprenorphine Mean
SD1.25
0.5841.84
0.6210.93
3.94531.66
12.66Norbuprenorphine Mean
SD0.301
0.1272.36
2.7512.39
4.52639.28
20.858 mgb Buprenorphine Mean
SD2.88
1.141.28
0.4628.39
10.2235.01
14.7Norbuprenorphine Mean
SD1.38
0.7521.75
2.1150.18
22.6144.33
19.2716 mgc Buprenorphine Mean
SD4.70
2.161.42
0.5047.09
20.0336.51
13.99Norbuprenorphine Mean
SD2.65
1.621.52
1.3492.31
34.7440.35
12.07Distribution: Buprenorphine is approximately 96% protein bound, primarily to alpha and beta globulin.
Metabolism: Buprenorphine undergoes both N-dealkylation to norbuprenorphine and glucuronidation. The N-dealkylation pathway is mediated primarily by CYP3A4. Norbuprenorphine, the major metabolite, can further undergo glucuronidation. Norbuprenorphine has been found to bind opioid receptors in-vitro; however, it has not been studied clinically for opioid-like activity.
Elimination: A mass balance study of buprenorphine showed complete recovery of radiolabel in urine (30%) and feces (69%) collected up to 11 days after dosing. Almost all of the dose was accounted for in terms of buprenorphine, norbuprenorphine, and two unidentified buprenorphine metabolites. In urine, most of buprenorphine and norbuprenorphine was conjugated (buprenorphine, 1% free and 9.4% conjugated; norbuprenorphine, 2.7% free and 11% conjugated). In feces, almost all of the buprenorphine and norbuprenorphine were free (buprenorphine, 33% free and 5% conjugated; norbuprenorphine, 21% free and 2% conjugated).
Buprenorphine has a mean elimination half-life from plasma ranging from 31 to 35 hours.
Drug-drug interactions: CYP3A4 Inhibitors and Inducers: Subjects receiving Buprenorphine Sublingual Tablets should be monitored if inhibitors of CYP3A4 such as azole antifungal agents (e.g., ketoconazole), macrolide antibiotics (e.g., erythromycin) or HIV protease inhibitors and may require dose-reduction of one or both agents. The interaction of buprenorphine with all CYP3A4 inducers has not been studied, therefore it is recommended that patients receiving Buprenorphine Sublingual Tablets be monitored for signs and symptoms of opioid withdrawal if inducers of CYP3A4 (e.g., phenobarbital, carbamazepine, phenytoin, rifampicin) are co-administered [see Drug Interactions (7.1)].
Buprenorphine has been found to be a CYP2D6 and CYP3A4 inhibitor and its major metabolite, norbuprenorphine has been found to be a moderate CYP2D6 inhibitor in in vitro studies employing human liver microsomes. However, the relatively low plasma concentrations of buprenorphine and norbuprenorphine resulting from therapeutic doses are not expected to raise significant drug-drug interaction concerns.
Special Populations
Hepatic Impairment: In a pharmacokinetic study, the disposition of buprenorphine was determined after administering a 2mg/0.5 mg buprenorphine and naloxone sublingual tablet in subjects with varied degrees of hepatic impairment as indicated by Child-Pugh criteria. The disposition of buprenorphine in patients with hepatic impairment was compared to disposition in subjects with normal hepatic function.In subjects with mild hepatic impairment, the changes in mean Cmax, AUC0-last, and half-life values of buprenorphine were not clinically significant. No dose adjustment is needed in patients with mild hepatic impairment.
For subjects with moderate and severe hepatic impairment, mean Cmax, AUC0-last, and half-life values of buprenorphine were increased (Table 4). [see Warnings and Precautions (5.12) and Use in Specific Populations (8.6)].
Table 4. Changes in Buprenorphine Pharmacokinetic Parameters in Subjects with Moderate and Severe Hepatic Impairment
Hepatic
ImpairmentPK Parameters
Increase in buprenorphine
compared to healthy subjectsModerate
Cmax
8%
AUC0-last
64%
Half-life
35%
Severe
Cmax
72%
AUC0-last
181%
Half-life
57%
HCV infection: In subjects with HCV infection but no sign of hepatic impairment, the changes in the mean Cmax, AUC0-last, and half-life values of buprenorphine were not clinically significant in comparison to healthy subjects without HCV infection. No dose adjustment is needed in patients with HCV infection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity: Carcinogenicity studies of buprenorphine were conducted in Sprague-Dawley rats and CD-1 mice. Buprenorphine was administered in the diet to rats at doses of 0.6, 5.5, and 56 mg/kg/day (estimated exposure was approximately 0.4, 3 and 35 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis) for 27 months. As in the buprenorphine/naloxone carcinogenicity study in rat, statistically significant dose-related increases in Leydig cell tumors occurred. In an 86-week study in CD-1 mice, buprenorphine was not carcinogenic at dietary doses up to 100 mg/kg/day (estimated exposure was approximately 30 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis).
Mutagenicity: Buprenorphine was studied in a series of tests utilizing gene, chromosome, and DNA interactions in both prokaryotic and eukaryotic systems. Results were negative in yeast (S. cerevisiae) for recombinant, gene convertant, or forward mutations; negative in Bacillus subtilis "rec" assay, negative for clastogenicity in CHO cells, Chinese hamster bone marrow and spermatogonia cells, and negative in the mouse lymphoma L5178Y assay.
Results were equivocal in the Ames test: negative in studies in two laboratories, but positive for frame shift mutation at a high dose (5 mg/plate) in a third study. Results were positive in the Green-Tweets (E. coli) survival test, positive in a DNA synthesis inhibition (DSI) test with testicular tissue from mice, for both in vivo and in vitro incorporation of [3H]thymidine, and positive in unscheduled DNA synthesis (UDS) test using testicular cells from mice.
Impairment of Fertility: Reproduction studies of buprenorphine in rats demonstrated no evidence of impaired fertility at daily oral doses up to 80 mg/kg/day (estimated exposure was approximately 50 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis) or up to 5 mg/kg/day IM or SC (estimated exposure was approximately 3 times the recommended human daily sublingual dose of 16 mg on a mg/m2 basis).
-
14 CLINICAL STUDIES
Clinical data on the safety and efficacy of buprenorphine were derived from studies of Buprenorphine Sublingual Tablet formulations, with and without naloxone, and from studies of sublingual administration of a more bioavailable ethanolic solution of buprenorphine.
Buprenorphine Sublingual Tablets were studied in 1834 patients; buprenorphine and naloxone tablets in 575 patients, and buprenorphine sublingual solutions in 2470 patients. A total of 1270 women received buprenorphine in those clinical trials. Dosing recommendations are based on data from one trial of both tablet formulations and two trials of the ethanolic solution. All trials used buprenorphine in conjunction with psychosocial counseling as part of a comprehensive addiction treatment program. There were no clinical studies conducted to assess the efficacy of buprenorphine as the only component of treatment.
In a double-blind placebo- and active-controlled study, 326 heroin-addicted subjects were randomly assigned to either buprenorphine and naloxone sublingual tablets, 16/4 mg per day; Buprenorphine Sublingual Tablets, 16 mg per day; or placebo sublingual tablets. For subjects randomized to either active treatment, dosing began with one 8 mg Buprenorphine on Day 1, followed by 16 mg (two 8 mg tablets) of buprenorphine on Day 2. On Day 3, those randomized to receive buprenorphine and naloxone sublingual tablets were switched to the combination tablet. Subjects randomized to placebo received one placebo tablet on Day 1 and two placebo tablets per day thereafter for four weeks. Subjects were seen daily in the clinic (Monday through Friday) for dosing and efficacy assessments. Take-home doses were provided for weekends. Subjects were instructed to hold the medication under the tongue for approximately 5 to 10 minutes until completely dissolved. Subjects received counseling regarding HIV infection and up to one hour of individualized counseling per week. The primary study comparison was to assess the efficacy of buprenorphine and naloxone sublingual tablets and Buprenorphine Sublingual Tablets individually against placebo sublingual tablet. The percentage of thrice-weekly urine samples that were negative for non-study opioids was statistically higher for both buprenorphine and naloxone sublingual tablets and Buprenorphine Sublingual Tablets than for placebo sublingual tablets.
In a double-blind, double-dummy, parallel-group study comparing buprenorphine ethanolic solution to a full agonist active control, 162 subjects were randomized to receive the ethanolic sublingual solution of buprenorphine at 8 mg/day (a dose which is roughly comparable to a dose of 12 mg per day of Buprenorphine Sublingual Tablets), or two relatively low doses of active control, one of which was low enough to serve as an alternative to placebo, during a 3 to 10 day induction phase, a 16-week maintenance phase and a 7-week detoxification phase. Buprenorphine was titrated to maintenance dose by Day 3; active control doses were titrated more gradually.
Maintenance dosing continued through Week 17, and then medications were tapered by approximately 20% to 30% per week over Weeks 18 to 24, with placebo dosing for the last two weeks. Subjects received individual and/or group counseling weekly.
Based on retention in treatment and the percentage of thrice-weekly urine samples negative for non-study opioids, buprenorphine was more effective than the low dose of the control, in keeping heroin addicts in treatment and in reducing their use of opioids while in treatment. The effectiveness of buprenorphine, 8 mg per day was similar to that of the moderate active control dose, but equivalence was not demonstrated.
In a dose-controlled, double-blind, parallel-group, 16-week study, 731 subjects were randomized to receive one of four doses of buprenorphine ethanolic solution: 1 mg, 4 mg, 8 mg, and 16 mg. Buprenorphine was titrated to maintenance doses over 1 to 4 days and continued for 16 weeks. Subjects received at least one session of AIDS education and additional counseling ranging from one hour per month to one hour per week, depending on site.
Based on retention in treatment and the percentage of thrice-weekly urine samples negative for non-study opioids, the three highest tested doses were superior to the 1 mg dose. Therefore, this study showed that a range of buprenorphine doses may be effective. The 1 mg dose of buprenorphine sublingual solution can be considered to be somewhat lower than a 2 mg tablet dose. The other doses used in the study encompass a range of tablet doses from approximately 6 mg to approximately 24 mg.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Buprenorphine Sublingual Tablets are supplied in white HDPE bottles.
8 mg - White, round, biconvex uncoated tablets with "8" debossed on one side and a dart "→" debossed on the other side
NDC: 53217-246-30 30 tablets per bottle Repackaged by
Aidarex Pharmaceuticals, LLC
Corona, CA 92880
Storage
Store at 25°C (77°F), excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in USP.
CAUTION: DEA Order Form Required.
Patients should be advised to store buprenorphine-containing medications safely and out of sight and reach of children.
Destroy any unused medication appropriately. [see Patient Counseling (17)]. -
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
Safe Use
Before initiating treatment with Buprenorphine Sublingual Tablets, explain the points listed below to caregivers and patients. Instruct patients to read the Medication Guide each time Buprenorphine Sublingual Tablets are dispensed because new information may be available.- Patients should be warned that it is extremely dangerous to self-administer non-prescribed benzodiazepines or other CNS depressants (including alcohol) while taking Buprenorphine Sublingual Tablets. Patients prescribed benzodiazepines or other CNS depressants should be cautioned to use them only as directed by their physicians. [see Warnings and Precautions (5.2), Drug Interactions (7.3)].
- Patients should be advised that Buprenorphine Sublingual Tablets contains an opioid that can be a target for people who abuse prescription medications or street drugs. Patients should be cautioned to keep their tablets in a safe place, and to protect them from theft.
- Patients should be instructed to keep Buprenorphine Sublingual Tablets in a secure place, out of the sight and reach of children. Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death. Patients should be advised that if a child is exposed to Buprenorphine Sublingual Tablets, medical attention should be sought immediately.
- Inform patients that Buprenorphine Sublingual Tablets could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their physicians if they are taking, or plan to take serotonergic medications [see Drug Interactions (7.4].
- Inform patients that Buprenorphine Sublingual Tablets could cause adrenal insufficiency, a potentially life-threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms [see Warnings and Precautions (5.6)].
- Patients should be advised never to give Buprenorphine Sublingual Tablets to anyone else, even if he or she has the same signs and symptoms. It may cause harm or death.
- Patients should be advised that selling or giving away this medication is against the law.
- Patients should be cautioned that Buprenorphine Sublingual Tablets may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving or operating hazardous machinery. Caution should be taken especially during drug induction and dose adjustment and until individuals are reasonably certain that buprenorphine therapy does not adversely affect their ability to engage in such activities. [see Warnings and Precautions (5.13)].
- Patients should be advised not to change the dosage of Buprenorphine Sublingual Tablets without consulting their physicians.
- Patients should be advised to take Buprenorphine Sublingual Tablets once a day.
- Patients should be informed that Buprenorphine Sublingual Tablets can cause drug dependence and that withdrawal signs and symptoms may occur when the medication is discontinued.
- Patients seeking to discontinue treatment with buprenorphine for opioid dependence should be advised to work closely with their physicians on a tapering schedule and should be apprised of the potential to relapse to illicit drug use associated with discontinuation of opioid agonist/partial agonist medication-assisted treatment.
- Patients should be cautioned that, like other opioids, Buprenorphine Sublingual Tablets may produce orthostatic hypotension in ambulatory individuals. [see Warnings and Precautions (5.12)].
- Patients should inform their physicians if any other prescription medications, over-the-counter medications, or herbal preparations are prescribed or currently being used. [see Drug Interactions (7.1, 7.2 and 7.3)].
- Advise women that if they are pregnant while being treated with Buprenorphine Sublingual Tablets, the baby may have signs of withdrawal at birth and that withdrawal is treatable [see Warnings and Precautions (5.5), Specific Populations (8.1)].
- Patients should be warned that buprenorphine passes into breast milk. Breast-feeding is therefore not advised in mothers treated with buprenorphine products. [see Specific Populations (8.3)].
- Patients should inform their family members that, in the event of emergency, the treating physician or emergency room staff should be informed that the patient is physically dependent on an opioid and that the patient is being treated with Buprenorphine Sublingual Tablets.
- Refer to the Medication Guide for additional information regarding the counseling information.
Disposal of Unused Buprenorphine Sublingual Tablets
Unused Buprenorphine Sublingual Tablets should be disposed of as soon as they are no longer needed. Flush unused tablets down the toilet. -
MEDICATION GUIDE
MEDICATION GUIDE
BUPRENORPHINE (byoo-pre-NOR-feen) SUBLINGUAL TABLETS CIII
Rx Only
IMPORTANT:
Keep Buprenorphine Sublingual Tablets in a secure place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally uses Buprenorphine Sublingual Tablets, get emergency help right away.Read this Medication Guide before you start taking Buprenorphine Sublingual Tablets and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor. Talk to your doctor or pharmacist if you have questions about Buprenorphine Sublingual Tablets.
Share the important information in this Medication Guide with members of your household.
What is the most important information I should know about Buprenorphine Sublingual Tablets?
- Buprenorphine Sublingual Tablets can cause serious and
life-threatening breathing problems. Call your doctor right
away or get emergency help if:
- You feel faint, dizzy or confused
- Your breathing gets much slower than is normal for you
These can be signs of an overdose or other serious problems.
- Buprenorphine Sublingual Tablets contains an opioid
that can cause physical dependence.
- Do not stop taking Buprenorphine Sublingual Tablets without talking to your doctor. You could become sick with uncomfortable withdrawal signs and symptoms because your body has become used to this medicine.
- Physical dependence is not the same as drug addiction
- Buprenorphine Sublingual Tablets are not for occasional or "as needed" use
- An overdose, and even death, can happen if you take benzodiazepines, sedatives, tranquilizers, or alcohol while using Buprenorphine Sublingual Tablets. Ask your doctor what you should do if you are taking one of these.
- Call a doctor or get emergency help right away if
you:
- Feel sleepy and uncoordinated
- Have blurred vision
- Have slurred speech
- Cannot think well or clearly
- Have slowed reflexes and breathing
- Do not inject ("shoot-up") Buprenorphine
Sublingual Tablets.
- Injecting this medicine may cause life-threatening infections and other serious health problems
- Injecting Buprenorphine Sublingual Tablets may cause serious withdrawal symptoms such as pain, cramps, vomiting, diarrhea, anxiety, sleep problems and cravings
- In an emergency, have family members tell the emergency department staff that you are physically dependent on an opioid and are being treated with Buprenorphine Sublingual Tablets .
What is Buprenorphine Sublingual Tablets?
- Buprenorphine Sublingual Tablets is a prescription medicine used to begin treatment in adults who are addicted to (dependent on) opioid drugs (either prescription or illegal), as part of a complete treatment program that also includes counseling and behavioral therapy.
- Buprenorphine Sublingual Tablets are most often used for the first 1 or 2 days to help you start with treatment.
Buprenorphine Sublingual Tablets is a controlled substance (CIII) because it contains buprenorphine, which can be a target for people who abuse prescription medicines or street drugs. Keep your Buprenorphine Sublingual Tablets in a safe place to protect them from theft. Never give your Buprenorphine Sublingual Tablets to anyone else; they can cause death or harm them. Selling or giving away this medicine is against the law.
- It is not known if Buprenorphine Sublingual Tablets is safe or effective in children.
Who should not take Buprenorphine Sublingual Tablets?
Do not take Buprenorphine Sublingual Tablets if you are allergic to buprenorphine.
What should I tell my doctor before taking Buprenorphine Sublingual Tablets?
Buprenorphine Sublingual Tablets may not be right for you. Before taking Buprenorphine Sublingual Tablets, tell your doctor if you:
- Have trouble breathing or lung problems
- Have an enlarged prostate gland (men)
- Have a head injury or brain problem
- Have problems urinating
- Have a curve in your spine that affects your breathing
- Have liver or kidney problems
- Have gallbladder problems
- Have adrenal gland problems
- Have Addison's disease
- Have low thyroid (hypothyroidism)
- Have a history of alcoholism
- Have mental problems such as hallucinations (seeing or hearing things that are not there)
- Have any other medical condition
- Are pregnant or plan to become pregnant. If you take Buprenorphine Sublingual Tablets while pregnant, your baby may have symptoms of opioid withdrawal or respiratory depression at birth. Talk to your doctor if you are pregnant or plan to become pregnant.
- Are breastfeeding or plan to breastfeed. Buprenorphine hydrochloride can pass into your milk and may harm the baby. Talk to your doctor about the best way to feed your baby if you take Buprenorphine Sublingual Tablets. Breastfeeding is not recommended while taking Buprenorphine Sublingual Tablets.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal supplements. Buprenorphine Sublingual Tablets may affect the way other medicines work, and other medicines may affect how Buprenorphine Sublingual Tablets works. Some medicines may cause serious or life-threatening medical problems when taken with Buprenorphine Sublingual Tablets.
Sometimes the doses of certain medicines and Buprenorphine Sublingual Tablets may need to be changed if used together. Do not take any medicine while using Buprenorphine Sublingual Tablets until you have talked with your doctor. Your doctor will tell you if it is safe to take other medicines while you are using Buprenorphine Sublingual Tablets.
Be especially careful about taking other medicines that may make you sleepy, such as pain medicines, tranquilizers, sleeping pills, anxiety medicines or antihistamines.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist each time you get a new medicine.
How should I take Buprenorphine Sublingual Tablets?
- Always take Buprenorphine Sublingual Tablets exactly as your doctor tells you. Your doctor may change your dose after seeing how it affects you. Do not change your dose unless your doctor tells you to change it.
- Do not take Buprenorphine Sublingual Tablets more often than prescribed by your doctor.
- If you are prescribed a dose of 2 or more Buprenorphine
Sublingual Tablets at the same time:
- Ask your doctor for instructions on the right way to take Buprenorphine Sublingual Tablets
- Follow the same instructions every time you take a dose of Buprenorphine Sublingual Tablets
- Put the tablets under your tongue. Let them dissolve
completely.
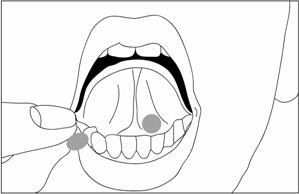
- While Buprenorphine Sublingual Tablets is dissolving, do not chew or swallow the tablet because the medicine will not work as well.
- Talking while the tablet is dissolving can affect how well the medicine in Buprenorphine Sublingual Tablets is absorbed.
- If you miss a dose of Buprenorphine Sublingual Tablets, take your medicine when you remember. If it is almost time for your next dose, skip the missed dose and take the next dose at your regular time. Do not take 2 doses at the same time unless your doctor tells you to. If you are not sure about your dosing, call your doctor.
- Do not stop taking Buprenorphine Sublingual Tablets suddenly. You could become sick and have withdrawal symptoms because your body has become used to the medicine. Physical dependence is not the same as drug addiction. Your doctor can tell you more about the differences between physical dependence and drug addiction. To have fewer withdrawal symptoms, ask your doctor how to stop using Buprenorphine Sublingual Tablets the right way.
- If you take too many Buprenorphine Sublingual Tablets or overdose, call Poison Control or get emergency medical help right away.
What should I avoid while taking Buprenorphine Sublingual Tablets?
- Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how this medication affects you. Buprenorphine can cause drowsiness and slow reaction times. This may happen more often in the first few weeks of treatment when your dose is being changed, but can also happen if you drink alcohol or take other sedative drugs when you take Buprenorphine Sublingual Tablets.
- You should not drink alcohol while using Buprenorphine Sublingual Tablets, as this can lead to loss of consciousness or death.
What are the possible side effects of Buprenorphine Sublingual Tablets?
Buprenorphine Sublingual Tablets can cause serious side effects including:
- See "What is the most important information I should know about Buprenorphine Sublingual Tablets?"
- Respiratory problems. You have a higher risk of death and coma if you take Buprenorphine Sublingual Tablets with other medicines, such as benzodiazepines.
- Sleepiness, dizziness, and problems with coordination
- Dependency or abuse
- Liver problems. Call your doctor right away if you notice any of these signs of liver problems: Your skin or the white part of your eyes turning yellow (jaundice), urine turning dark, stools turning light in color, you have less of an appetite, or you have stomach (abdominal) pain or nausea. Your doctor should do tests before you start taking and while you take Buprenorphine Sublingual Tablets.
- Allergic reaction. You may have a rash, hives, swelling of your face, wheezing, or loss of blood pressure and consciousness. Call a doctor or get emergency help right away.
- Opioid withdrawal. This can include: shaking, sweating more than normal, feeling hot or cold more than normal, runny nose, watery eyes, goose bumps, diarrhea, vomiting and muscle aches. Tell your doctor if you develop any of these symptoms.
- Decrease in blood pressure. You may feel dizzy if you get up too fast from sitting or lying down.
Common side effects of Buprenorphine Sublingual Tablets include:
- Headache
- Nausea
- Vomiting
- Increased sweating
- Constipation
- Drug withdrawal syndrome
- Decrease in sleep (insomnia)
- Pain
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all the possible side effects of Buprenorphine Sublingual Tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Buprenorphine Sublingual Tablets?
- Store Buprenorphine Sublingual Tablets between 59°F and 86°F (15°C to 30°C)
- Keep Buprenorphine Sublingual Tablets in a safe place, out of the sight and reach of children
How should I dispose of unused Buprenorphine Sublingual Tablets?
- Dispose of unused Buprenorphine Sublingual Tablets as soon as you no longer need them.
- Flush unused tablets down the toilet.
General information about the safe and effective use of Buprenorphine Sublingual Tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Buprenorphine Sublingual Tablets for a condition for which they were not prescribed. Do not give Buprenorphine Sublingual Tablets to other people, even if they have the same symptoms you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about Buprenorphine Sublingual Tablets. If you would like more information, talk to your doctor or pharmacist. You can ask your doctor or pharmacist for information that is written for healthcare professionals.
For more information call Hi-Tech Pharmacal Co., Inc. at 1-888-775-1770.
What are the ingredients in Buprenorphine Sublingual Tablets?
Active Ingredient: buprenorphine hydrochloride
Inactive ingredient: citric acid, cornstarch, lactose monohydrate, mannitol, povidone K30, sodium citrate anhydrous and sodium stearyl fumerate
Manufactured by:
Ethypharm S.A.
76121 Le Grand Quevilly Cedex
FranceDistributed by:
Hi-Tech Pharmacal Co., Inc.
Amityville, NY 11701
Tel: 1-888-775-1770
www.hitechpharm.com/drugsafetyThis Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: November 2016.
- Buprenorphine Sublingual Tablets can cause serious and
life-threatening breathing problems. Call your doctor right
away or get emergency help if:
- PRINCIPAL DISPLAY PANEL - 8 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
BUPRENORPHINE HYDROCHLORIDE
buprenorphine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 53217-246(NDC:50383-930) Route of Administration SUBLINGUAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 8 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) 37.79 mg SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MANNITOL (UNII: 3OWL53L36A) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) POVIDONE K30 (UNII: U725QWY32X) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code 8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53217-246-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/24/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090622 09/24/2010 Labeler - Aidarex Pharmaceuticals LLC (801503249)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.