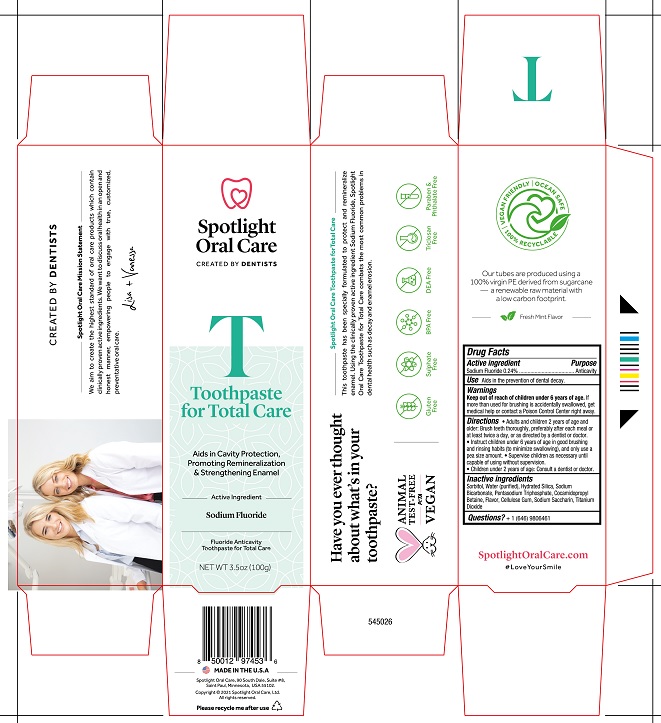
Spotlight Oral Care Toothpaste for Total Care
Spotlight Oral Care for Total Care by
Drug Labeling and Warnings
Spotlight Oral Care for Total Care by is a Otc medication manufactured, distributed, or labeled by Oral Spotlight Care Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPOTLIGHT ORAL CARE FOR TOTAL CARE- sodium fluoride paste
Oral Spotlight Care Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Spotlight Oral Care Toothpaste for Total Care
Keep out of reach of children under 6 years of age. If
more than used for brushing is accidentally swallowed, get
medical help or contact a Poison Control Center right away.
Directions Adults and children 2 years of age and
older: Brush teeth vithouroughly, preferably after each meal or
at least twice a day, or as directed by a dentist or doctor.
Instruct children under 6 years of age in good brushing
and rinsing habits (to minimize swallowing), and only use a
pea size amount Supervise children as necessary until
capable of using without supervision.
Children under 2 years of age: Consult a dentist or doctor.
Inactive Ingredients
Sorbitol, Water (purified), Hydrated Silica, Sodium
Bicarbonate, Pentasodium Triphosphate, Cocamidopropyl
Betaine, Flavor, Cellulose Gum. Sodium Saccharin, Titanium
Dioxide
Product Labeling
Spotlight
Oral Care
CREATED BY DENTISTS
T
Toothpaste
for Total
Care
_______ Active Ingredient _________
Sodium Fluoride
______________________________
NET WT 3.5oz (100g)
MADE IN THE USA
Spotlight Oral Care, 90 South Dale, Suite #8
Saint Paul, Minnesota, USA 55102
SpotlightOralCare.com
#LoveYourSmile
Carton
Tube

res
| SPOTLIGHT ORAL CARE FOR TOTAL CARE
sodium fluoride paste |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Oral Spotlight Care Inc (117405870) |
| Registrant - Oral Spotlight Care Inc (117405870) |