CARBIDOPA AND LEVODOPA capsule, extended release
Carbidopa and Levodopa by
Drug Labeling and Warnings
Carbidopa and Levodopa by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals USA Inc., Amneal Pharmaceuticals Pvt. Ltd., Bora Pharmaceutical Laboratories Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CARBIDOPA AND LEVODOPA EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for CARBIDOPA AND LEVODOPA EXTENDED-RELEASE CAPSULES.
CARBIDOPA and LEVODOPA extended-release capsules, for oral use
Initial U.S. Approval: 1975INDICATIONS AND USAGE
Carbidopa and levodopa extended-release capsule is a combination of carbidopa (an aromatic amino acid decarboxylation inhibitor) and levodopa (an aromatic amino acid) indicated for the treatment of Parkinson's disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication or manganese intoxication (1)
DOSAGE AND ADMINISTRATION
- Levodopa-naïve patients: Starting dose is 23.75 mg / 95 mg three times daily; may increase to 36.25 mg / 145 mg three times daily on the fourth day of treatment (2.1)
- See Table 1 for instructions for converting patients taking immediate-release carbidopa-levodopa to an initial dose of carbidopa and levodopa extended-release capsules. Dosages of carbidopa and levodopa extended-release capsules are not interchangeable with other carbidopa-levodopa products (2.2)
- The maximum recommended daily dose of carbidopa and levodopa extended-release capsules is 612.5 mg/2,450 mg (2.1, 2.2)
- Carbidopa and levodopa extended-release capsules may be taken with or without food; do not chew, divide or crush (2.4, 12.3)
DOSAGE FORMS AND STRENGTHS
Extended-release capsules: Carbidopa and levodopa 23.75 mg/95 mg, 36.25 mg/145 mg, 48.75 mg/195 mg, 61.25 mg/245 mg (3)
CONTRAINDICATIONS
- Nonselective MAO inhibitors (4)
WARNINGS AND PRECAUTIONS
- May cause falling asleep during activities of daily living (5.1)
- Avoid sudden discontinuation or rapid dose reduction to reduce the risk of withdrawal-emergent hyperpyrexia and confusion (5.2)
- Cardiovascular Events: Monitor patients with a history of cardiovascular disease (5.3)
- Hallucinations/Psychosis may occur (5.4)
- Impulse Control Disorders: Consider dose reduction or stopping carbidopa and levodopa extended-release capsules if occurs (5.5)
- May cause or exacerbate dyskinesia: Consider dose reduction (5.6)
ADVERSE REACTIONS
- Early Parkinson's disease: Most common adverse reactions (incidence ≥ 5% and greater than placebo) are nausea, dizziness, headache, insomnia, abnormal dreams, dry mouth, dyskinesia, anxiety, constipation, vomiting, and orthostatic hypotension (6.1)
- Advanced Parkinson's disease: Most common adverse reactions (incidence ≥ 5% and greater than oral immediate-release carbidopa-levodopa) are nausea and headache (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Patients Naïve to Levodopa Therapy
2.2 Converting from Immediate-Release Carbidopa-Levodopa to Carbidopa and Levodopa Extended-Release Capsules
2.3 Discontinuation of Carbidopa And Levodopa Extended-Release Capsules
2.4 Administration Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
5.2 Withdrawal-Emergent Hyperpyrexia and Confusion
5.3 Cardiovascular Ischemic Events
5.4 Hallucinations/Psychosis
5.5 Impulse Control/Compulsive Behaviors
5.6 Dyskinesia
5.7 Peptic Ulcer Disease
5.8 Glaucoma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
7.2 Dopamine D2 Receptor Antagonists and Isoniazid
7.3 Iron Salts
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Patients with Early Parkinson’s Disease
14.2 Patients with Advanced Parkinson’s Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Patients Naïve to Levodopa Therapy
The recommended starting dosage of carbidopa and levodopa extended-release capsules in levodopa-naïve patients is 23.75 mg/95 mg taken orally three times a day for the first 3 days. On the fourth day of treatment, the dosage of carbidopa and levodopa extended-release capsules may be increased to 36.25 mg/145 mg taken three times a day.
Based upon individual patient clinical response and tolerability, the carbidopa and levodopa extended-release capsules dose may be increased up to a maximum recommended dose of 97.5 mg/390 mg taken three times a day. The dosing frequency may be changed from three times a day to a maximum of five times a day if more frequent dosing is needed and if tolerated.
Maintain patients on the lowest dosage required to achieve symptomatic control and to minimize adverse reactions such as dyskinesia and nausea. The maximum recommended daily dose of carbidopa and levodopa extended-release capsules is 612.5 mg/2,450 mg.
2.2 Converting from Immediate-Release Carbidopa-Levodopa to Carbidopa and Levodopa Extended-Release Capsules
The dosages of other carbidopa and levodopa products are not interchangeable on a 1:1 basis with the dosages of carbidopa and levodopa extended-release capsules.
To convert patients from immediate-release carbidopa-levodopa to carbidopa and levodopa extended-release capsules, first calculate the patient's current total daily dose of levodopa. The starting total daily dose of carbidopa and levodopa extended-release capsules is as recommended in Table 1.
After conversion, any combination of the four carbidopa and levodopa extended-release capsules dosage strengths can be used to achieve an optimal dosing. Adjust the dose and dosing frequency as necessary to maintain patient tolerance and sufficient symptomatic control. Administration of concomitant Parkinson's disease medications should remain stable while adjusting the carbidopa and levodopa extended-release capsules dose. In clinical trials, carbidopa and levodopa extended-release capsules were administered in divided doses of three to five times a day. The maximum recommended total daily dose of carbidopa and levodopa extended-release capsule is 612.5 mg/2,450 mg.
For patients currently treated with carbidopa and levodopa plus a catechol-O-methyl transferase (COMT) inhibitor (such as entacapone), the initial total daily dose of levodopa in carbidopa and levodopa extended-release capsules described in Table 1 may need to be increased.
Use of carbidopa and levodopa extended-release capsules in combination with other levodopa products has not been studied.
Table 1
Conversion from Immediate-Release Carbidopa-Levodopa to
Carbidopa and Levodopa Extended-Release Capsules
Total Daily Dose of
Levodopa in
Immediate-Release
Carbidopa-
Levodopa
Recommended Starting Dosage of Carbidopa and Levodopa Extended-Release Capsules
Total Daily Dose of Levodopa in
Carbidopa and Levodopa Extended-Release Capsules
Carbidopa and Levodopa Extended-Release Capsules Dosing Regimen
400 mg to 549 mg
855 mg
3 capsules carbidopa and levodopa extended-release capsules 23.75 mg/95 mg taken TIDa
550 mg to 749 mg
1,140 mg
4 capsules carbidopa and levodopa extended-release capsules 23.75 mg/95 mg taken TID
750 mg to 949 mg
1,305 mg
3 capsules carbidopa and levodopa extended-release capsules 36.25 mg/145 mg taken TID
950 mg to 1,249 mg
1,755 mg
3 capsules carbidopa and levodopa extended-release capsules 48.75 mg/195 mg taken TID
Equal to or greater
than 1,250 mg
2,340 mg
or
4 capsules carbidopa and levodopa extended-release capsules 48.75 mg/195 mg taken TID
or
2,205 mg
3 capsules carbidopa and levodopa extended-release capsules 61.25 mg/245 mg taken TID
2.3 Discontinuation of Carbidopa And Levodopa Extended-Release Capsules
Avoid sudden discontinuation or rapid dose reduction of carbidopa and levodopa extended-release capsules. The daily dose of carbidopa and levodopa extended-release capsules should be tapered at the time of treatment discontinuation [see Warnings and Precautions (5.2)].
2.4 Administration Information
Swallow carbidopa and levodopa extended-release capsules whole with or without food. A high-fat, high-calorie meal may delay the absorption of levodopa by about 2 hours [see Clinical Pharmacology (12.3)].
Do not chew, divide or crush carbidopa and levodopa extended-release capsules. For patients who have difficulty swallowing intact capsules, administer carbidopa and levodopa extended-release capsules by carefully twisting apart both halves of the capsule. Sprinkle the entire contents of both halves of the capsule on a small amount of applesauce (1 to 2 tablespoons) and consume the mixture immediately. Do not store the drug/food mixture for future use.
-
3 DOSAGE FORMS AND STRENGTHS
Carbidopa and Levodopa Extended-release Capsules, 23.75 mg/95 mg: blue and white capsule imprinted with IPX066 on the capsule cap and 95 on the capsule body.
Carbidopa and Levodopa Extended-release Capsules, 36.25 mg/145 mg: blue and light blue capsule imprinted with IPX066 on the capsule cap and 145 on the capsule body.
Carbidopa and Levodopa Extended-release Capsules, 48.75 mg/195 mg: blue and yellow capsule imprinted with IPX066 on the capsule cap and 195 on the capsule body.
Carbidopa and Levodopa Extended-release Capsules, 61.25 mg/245 mg: blue capsule imprinted with IPX066 on the capsule cap and 245 on the capsule body.
-
4 CONTRAINDICATIONS
Carbidopa and levodopa extended-release capsules are contraindicated in patients:
- Currently taking a nonselective monoamine oxidase (MAO) inhibitor (e.g., phenelzine and tranylcypromine) or have recently (within 2 weeks) taken a nonselective MAO inhibitor. Hypertension can occur if these drugs are used concurrently [see Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
Patients treated with levodopa, a component of carbidopa and levodopa extended-release capsules, have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence while on levodopa, some perceived that they had no warning signs (sleep attack), such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events have been reported more than 1 year after initiation of treatment.
It has been reported that falling asleep while engaged in activities of daily living usually occurs in a setting of pre-existing somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness in carbidopa and levodopa extended-release capsules-treated patients, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with carbidopa and levodopa extended-release capsules, advise patients of the potential to develop drowsiness and specifically ask about factors that may increase the risk for somnolence with carbidopa and levodopa extended-release capsules such as concomitant sedating medications or the presence of a sleep disorder. Consider discontinuing carbidopa and levodopa extended-release capsules in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.).
If a decision is made to continue carbidopa and levodopa extended-release capsules, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if the patients become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.2 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex that resembles neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in dopaminergic therapy. Avoid sudden discontinuation or rapid dose reduction in patients taking carbidopa and levodopa extended-release capsules. If the decision is made to discontinue carbidopa and levodopa extended-release capsules, the dose should be tapered to reduce the risk of hyperpyrexia and confusion [see Dosage and Administration (2.4)].
5.3 Cardiovascular Ischemic Events
Cardiovascular ischemic events have occurred in patients taking carbidopa and levodopa extended-release capsules. In a placebo controlled clinical study in patients with early Parkinson's disease, 7/289 (2.4%) of carbidopa and levodopa extended-release capsules-treated patients experienced cardiovascular ischemic adverse reactions compared to 1/92 (1.1%) of placebo-treated patients. In an active-controlled clinical study in patients with advanced Parkinson's disease, 3/450 (0.7%) of carbidopa and levodopa extended-release capsules-treated patients experienced cardiovascular ischemic adverse reactions compared to 0/471 oral immediate-release carbidopa-levodopa-treated patients. These patients all had a previous history of ischemic heart disease or risk factors for ischemic heart disease.
In patients with a history of myocardial infarction who have residual atrial, nodal, or ventricular arrhythmias, cardiac function should be monitored in an intensive cardiac care facility during the period of initial dosage adjustment.
5.4 Hallucinations/Psychosis
There is an increased risk for hallucinations and psychosis in patients taking carbidopa and levodopa extended-release capsules. In a controlled clinical trial in patients with advanced Parkinson's disease, 9/201 (4%) of carbidopa and levodopa extended-release capsules-treated patients reported hallucinations or psychosis compared to 2/192 (1%) of oral immediate-release carbidopa-levodopa-treated patients.
Hallucinations present shortly after the initiation of therapy and may be responsive to dose reduction in levodopa. Hallucinations may be accompanied by confusion, insomnia, and excessive dreaming. Abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Because of the risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with carbidopa and levodopa extended-release capsules. In addition, medications that antagonize the effects of dopamine used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of carbidopa and levodopa extended-release capsules [see Drug Interactions (7.2)].
5.5 Impulse Control/Compulsive Behaviors
Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including carbidopa and levodopa extended-release capsules, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued.
Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with carbidopa and levodopa extended-release capsules. Consider a dose reduction or stopping the medication if a patient develops such urges while taking carbidopa and levodopa extended-release capsules.
5.6 Dyskinesia
Carbidopa and levodopa extended-release capsules can cause dyskinesias that may require a dosage reduction of carbidopa and levodopa extended-release capsules or other medications used for the treatment of Parkinson's disease.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.1)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.2)]
- Cardiovascular Ischemic Events [see Warnings and Precautions (5.3)]
- Hallucinations/Psychosis [see Warnings and Precautions (5.4)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.5)]
- Dyskinesia [see Warnings and Precautions (5.6)]
- Peptic Ulcer Disease [see Warnings and Precautions (5.7)]
- Glaucoma [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety population consisted of a total of 978 Parkinson's disease patients who received at least one dose of carbidopa and levodopa extended-release capsules, and had an average duration of exposure of 40 weeks.
Adverse Reactions in Early Parkinson's Disease
In a placebo-controlled clinical study in patients with early Parkinson's disease (Study 1), the most common adverse reactions with carbidopa and levodopa extended-release capsules (in at least 5% of patients and more frequently than in placebo) were nausea, dizziness, headache, insomnia, abnormal dreams, dry mouth, dyskinesia, anxiety, constipation, vomiting, and orthostatic hypotension.
Table 2 lists adverse reactions occurring in at least 5% of carbidopa and levodopa extended-release capsules-treated patients and at a higher rate than placebo in Study 1.
Table 2
Adverse Reactions in Study 1 in Patients with Early Stage Parkinson's Disease
Placebo
Carbidopa and Levodopa Extended-release Capsules
36.25 mg Carbidopa
Carbidopa and Levodopa Extended-release Capsules
61.25 mg Carbidopa
Carbidopa and Levodopa Extended-release Capsules
97.5 mg Carbidopa
145 mg Levodopa TID
245 mg Levodopa TID
390 mg Levodopa TID
(N=92)
(N=87)
(N=104)
(N=98)
%
%
%
%
Nausea
9
14
19
20
Dizziness
5
9
19
12
Headache
11
7
13
17
Insomnia
3
2
9
6
Abnormal Dreams
0
2
6
5
Dry Mouth
1
3
2
7
Dyskinesia
0
2
4
5
Anxiety
0
2
3
5
Constipation
1
2
6
2
Vomiting
3
2
2
5
Orthostatic Hypotension
1
1
1
5
Adverse Reactions Leading to Discontinuation in Study 1
In Study 1, 12% of patients discontinued carbidopa and levodopa extended-release capsules early due to adverse reactions; a higher proportion of patients in the 61.25 mg/ 245 mg carbidopa and levodopa extended-release capsules-treated group (14%) and in the 97.5 mg/390 mg carbidopa and levodopa extended-release capsules-treated group (15%) experienced adverse reactions leading to early discontinuation compared to (4%) in the placebo group. The most common adverse reactions resulting in early discontinuation were nausea, dizziness, and vomiting.
Adverse Reactions in Advanced Parkinson's Disease
In an active-controlled clinical study in patients with advanced Parkinson's disease (Study 2), the most common adverse reactions with carbidopa and levodopa extended-release capsules that occurred during dose conversion or maintenance (in at least 5% of patients and more frequently than on oral immediate-release carbidopa-levodopa) were nausea and headache.
Table 3 lists adverse reactions occurring in at least 5% of carbidopa and levodopa extended-release capsules-treated patients and at a higher rate than oral immediate-release carbidopa-levodopa in Study 2.
Table 3
Adverse Reactions in Study 2 in Patients with Advanced Parkinson's Disease
Carbidopa and Levodopa Extended-release Capsules
Immediate-Release
Carbidopa-Levodopa
(N=201)
(N=192)
Period
Dose Conversiona
Maintenance
Dose Conversiona
Maintenance
%
%
%
%
Nausea
4
3
6
2
Headache
5
1
3
2
aAll patients were converted to carbidopa and levodopa extended-release capsules in the open-label Dose Conversion period and then received randomized treatment during maintenance.
Adverse Reactions Leading to Discontinuation in Study 2
In Study 2, 5% of patients discontinued treatment due to adverse reactions during conversion to carbidopa and levodopa extended-release capsules. The common adverse reactions leading to discontinuation during dose conversion were dyskinesia, anxiety, dizziness, and on and off phenomenon.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of carbidopa and levodopa extended-release capsules. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish causal relationship to carbidopa and levodopa extended-release capsules exposure.
Psychiatric: Suicide attempt, suicidal ideation.
-
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
The use of nonselective MAO inhibitors with carbidopa and levodopa extended-release capsules is contraindicated [see Contraindications (4)]. Discontinue use of any nonselective MAO inhibitors at least two weeks prior to initiating carbidopa and levodopa extended-release capsules.
The use of selective MAO-B inhibitors (e.g., rasagiline and selegiline) with carbidopa and levodopa extended-release capsules may be associated with orthostatic hypotension. Monitor patients who are taking these drugs concurrently.
7.2 Dopamine D2 Receptor Antagonists and Isoniazid
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone, metoclopramide) and isoniazid may reduce the effectiveness of levodopa. Monitor patients for worsening Parkinson's symptoms.
7.3 Iron Salts
Iron salts or multivitamins containing iron salts can form chelates with levodopa and carbidopa and can cause a reduction in the bioavailability of carbidopa and levodopa extended-release capsules. If iron salts or multivitamins containing iron salts are co-administered with carbidopa and levodopa extended-release capsules, monitor patients for worsening Parkinson's symptoms.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate data on the developmental risk associated with the use of carbidopa and levodopa extended-release capsules in pregnant women. In animal studies, carbidopa-levodopa has been shown to be developmentally toxic (including teratogenic effects) at clinically relevant doses (see Data).
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
When administered to pregnant rabbits throughout organogenesis, carbidopa-levodopa caused both visceral and skeletal malformation in fetuses at all doses and ratios of carbidopa-levodopa tested. No teratogenic effects were observed when carbidopa-levodopa was administered to pregnant mice throughout organogenesis.
There was a decrease in the number of live pups delivered by rats receiving carbidopa-levodopa during organogenesis.
8.2 Lactation
Levodopa has been detected in human milk after administration of carbidopa-levodopa. There are no data on the presence of carbidopa in human milk, the effects of levodopa or carbidopa on the breastfed infant, or the effects on milk production. However, inhibition of lactation may occur because levodopa decreases secretion of prolactin in humans. Carbidopa is excreted in rat milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for carbidopa and levodopa extended-release capsules and any potential adverse effects on the breastfed infant from carbidopa and levodopa extended-release capsules or from the underlying maternal condition.
-
10 OVERDOSAGE
In the active-controlled clinical study, a patient accidentally ingested 4.68 grams of carbidopa/18.7 grams of levodopa contained in carbidopa and levodopa extended-release capsules over a 2-day period. The patient experienced acute psychosis and dyskinesias. The patient recovered and completed the study on a reduced dose of carbidopa and levodopa extended-release capsules.
Based on the limited available information, the acute symptoms of levodopa/dopa decarboxylase inhibitor overdosage can be expected to arise from dopaminergic overstimulation. Doses of a few grams may result in CNS disturbances, with an increasing likelihood of cardiovascular disturbance (e.g., hypotension, tachycardia) and more severe psychiatric problems at higher doses. An isolated report of rhabdomyolysis and another of transient renal insufficiency suggest that levodopa overdosage may give rise to systemic complications, secondary to dopaminergic overstimulation.
Monitor patients and provide supportive care. Patients should receive electrocardiographic monitoring for the development of arrhythmias; if needed, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs, increasing the risk of drug interactions (especially catechol-structured drugs) should be taken into consideration.
-
11 DESCRIPTION
Carbidopa and levodopa extended-release capsule is a combination of carbidopa, an inhibitor of aromatic amino acid decarboxylation, and levodopa, an aromatic amino acid, in extended-release capsules for oral use.
Carbidopa is a white to creamy white powder, slightly soluble in water, with a molecular weight of 244.2. It is designated chemically as (-)-L-α-hydrazino-3, 4-dihydroxy-α-methylhydrocinnamic acid monohydrate. Its molecular formula is C10H14N2O4 H2O and its structural formula is:
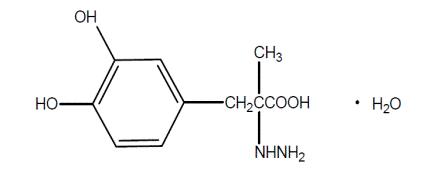
Capsule content is expressed in terms of anhydrous carbidopa, which has a molecular weight of 226.2.
Levodopa is a white to off-white, crystalline powder, slightly soluble in water, with a molecular weight of 197.2. It is designated chemically as (−)-3-(3, 4-Dihydroxyphenyl)-L-alanine. Its molecular formula is C9H11NO4 and its structural formula is:
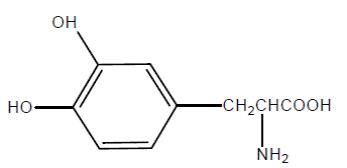
Each extended-release capsule contains 23.75 mg carbidopa, USP and 95 mg levodopa USP, 36.25 mg carbidopa, USP and 145 mg levodopa USP, 48.75 mg carbidopa, USP and 195 mg levodopa USP, or 61.25 mg carbidopa, USP and 245 mg levodopa USP. The inactive ingredients are microcrystalline cellulose, mannitol, tartaric acid, ethyl cellulose, hypromellose, sodium starch glycolate, sodium lauryl sulfate, povidone, talc, methacrylic acid copolymers, triethyl citrate, croscarmellose sodium, and magnesium stearate.
All capsule shells contain gelatin and titanium dioxide. In addition, all blue capsule shells contain FD&C Blue #2 and yellow iron oxide. All yellow capsule shells contain yellow iron oxide.
All capsules imprinted with blue pharmaceutical ink contain FD&C Blue #2, butyl alcohol, dehydrated alcohol, isopropyl alcohol, propylene glycol, shellac and strong ammonia solution.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
When levodopa is administered orally, it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. Carbidopa inhibits the decarboxylation of peripheral levodopa, making more levodopa available for delivery to the brain.
Levodopa
Levodopa is the metabolic precursor of dopamine, does cross the blood-brain barrier, and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson's disease.
12.2 Pharmacodynamics
Because its decarboxylase inhibiting activity is limited to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available to the brain. The addition of carbidopa to levodopa reduces the peripheral effects (nausea, vomiting) due to decarboxylation of levodopa; however, carbidopa does not decrease the adverse reactions due to the central effects of levodopa.
Patients treated with levodopa therapy for Parkinson's disease may develop motor fluctuations characterized by end-of-dose failure, peak dose dyskinesia, 'on-off' phenomenon, and akinesia.
12.3 Pharmacokinetics
Carbidopa
Following oral dosing of carbidopa and levodopa extended-release capsules the maximum concentration occurred at approximately 3 hours. The bioavailability of carbidopa from carbidopa and levodopa extended-release capsules relative to immediate-release carbidopa-levodopa tablets was approximately 50%.
Levodopa
The pharmacokinetics of carbidopa and levodopa extended-release capsules were evaluated following single-doses in healthy subjects and following single- and multiple-doses in patients with Parkinson's disease. The bioavailability of levodopa from carbidopa and levodopa extended-release capsules in patients was approximately 70% relative to immediate-release carbidopa-levodopa. For comparable doses, carbidopa and levodopa extended-release capsules results in a levodopa peak concentration (Cmax) that is 30% that of immediate-release carbidopa-levodopa. Following an initial peak at about one hour, plasma concentrations are maintained for about 4 to 5 hours before declining.
In patients with Parkinson's disease, multiple-dose pharmacokinetics was comparable to single-dose pharmacokinetics, i.e., there was minimal accumulation of levodopa. Variation in levodopa peak to trough plasma concentrations at steady-state defined as (Cmax-Cmin)/Cavg was approximately 1.5 for carbidopa and levodopa extended-release capsules compared to approximately 3.2 for immediate-release levodopa.
Distribution
Carbidopa is approximately 36% bound to plasma proteins. Approximately 10% to 30% of levodopa is bound to plasma protein.
Metabolism and Elimination
Carbidopa
The terminal phase elimination half-life of carbidopa is approximately 2 hours.
Carbidopa is metabolized to two main metabolites: α-methyl-3-methoxy-4-hydroxyphenylpropionic acid and α-methyl-3,4-dihydroxy-phenylpropionic acid. These two metabolites are primarily eliminated in the urine unchanged or as a glucuronide. Unchanged carbidopa accounts for 30% of the total urinary excretion.
Peripheral dopa-decarboxylase may be saturated by carbidopa in other carbidopa-levodopa products at 70 mg per day to 100 mg per day, which produces equivalent exposure to 140 mg to 200 mg of carbidopa provided by carbidopa and levodopa extended-release capsules.
Levodopa
The terminal phase elimination half-life of levodopa, the active moiety of antiparkinsonian activity, is approximately 2 hours in the presence of carbidopa.
Levodopa is extensively metabolized to various metabolites. The two major metabolic pathways are decarboxylation by dopa decarboxylase (DDC) and O-methylation by catechol-O-methyltransferase (COMT).
Dose Proportionality
Carbidopa and levodopa extended-release capsules show approximately dose proportional pharmacokinetics for both carbidopa and levodopa over the levodopa dosage strength range of 95 mg to 245 mg.
Effect of Food
In healthy adults, oral administration of carbidopa and levodopa extended-release capsules after a high-fat, high-calorie meal reduced Cmax approximately 21% and increased AUCinf approximately 13% for levodopa compared to administration in the fasted state. There may be a delay by 2 hours in the absorption of levodopa when carbidopa and levodopa extended-release capsule is taken with a high-fat, high-calorie meal. In addition, absorption of levodopa may be decreased by a high protein meal.
Specific Populations
Elderly
In pharmacokinetics studies following a single-dose of carbidopa and levodopa extended-release capsules, the peak concentrations of carbidopa and levodopa are generally similar between younger (45 to 60 years) and older (60 to 75 years) subjects.
Gender
In pharmacokinetics studies following a single-dose of carbidopa and levodopa extended-release capsules:
- Carbidopa
At comparable doses females are reported to have higher carbidopa peak concentrations and systemic exposure (approximately 33%) compared to males. Median time to peak concentration and terminal half-life are comparable between males and females.
- Levodopa
At comparable doses females are reported to have higher levodopa peak concentrations (approximately 23% to 33%) and systemic exposure (approximately 33% to 37%) compared to males. Median time to peak concentration and terminal half-life are comparable between males and females.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In rats, oral administration of carbidopa-levodopa for two years resulted in no evidence of carcinogenicity.
Mutagenesis
Carbidopa was mutagenic in the in vitro Ames test and in the in vitro mouse lymphoma tk assay but was negative in the in vivo mouse micronucleus assay.
Impairment of Fertility
In reproduction studies, no effects on fertility were observed in rats receiving carbidopa-
levodopa.
-
14 CLINICAL STUDIES
14.1 Patients with Early Parkinson’s Disease
The effectiveness of carbidopa and levodopa extended-release capsules in patients with early Parkinson's disease was established in a randomized, double-blind, placebo-controlled, fixed-dose, parallel-group, 30-week clinical trial (Study 1). Patients enrolled in Study 1 (n=381) were Hoehn and Yahr Stage I–III with a median disease duration of 1 year, and had limited or no prior exposure to levodopa and dopamine agonists. Patients continued taking concomitant selective monoamine oxidase B (MAO-B) inhibitors, amantadine, and anticholinergics provided the doses were stable for at least 4 weeks before screening. Eligible patients were randomized (1:1:1:1) to placebo or one of three fixed doses of carbidopa and levodopa extended-release capsules (carbidopa/levodopa doses of 36.25 mg/145 mg, 61.25 mg/245 mg, or 97.5 mg/390 mg, three times a day). Patients were not allowed to receive supplemental levodopa or catechol-O-methyl transferase (COMT) inhibitors. Patients receiving carbidopa and levodopa extended-release capsules initiated treatment at 23.75 mg/95 mg three times daily (TID). The dose was increased on Day 4 and the maximum study dose (97.5 mg/390 mg TID) was achieved by Day 22.
The clinical outcome measure in Study 1 was the mean change from baseline in the sum of the Unified Parkinson's Disease Rating Scale (UPDRS) Part II (activities of daily living) score, and UPDRS Part III (motor score) for carbidopa and levodopa extended-release capsules, compared to placebo at Week 30 (or early termination). The mean score decrease (i.e., improvement) from baseline to Week 30 for each of the three carbidopa and levodopa extended-release capsules dosage groups was significantly greater than for placebo. The results of Study 1 are shown in Table 4.
Table 4 Study 1: Change from Baseline in UPDRS Part II plus Part III Score at Week 30 (or at Early Termination) in Levodopa-Naïve Patients with Early Parkinson's Disease a For the UPDRS, higher scores indicate greater severity of impairment
b All values based on 361 patients who had valid End-of-Study values
c Negative numbers indicate improvement as compared with the baseline value
d P-value is less than 0.05
Mean UPDRS (Part II and Part III) Scorea
Treatment
Baselineb
Week 30
Change from Baseline
at Week 30c
Placebo
36.5
35.9
−0.6
Carbidopa and Levodopa Extended-release Capsules
36.25 mg / 145 mg TID
36.1
24.4
−11.7d
Carbidopa and Levodopa Extended-release Capsules
61.25 mg / 245 mg TID
38.2
25.3
−12.9d
Carbidopa and Levodopa Extended-release Capsules
97.5 mg / 390 mg TID
36.3
21.4
−14.9d
14.2 Patients with Advanced Parkinson’s Disease
Study 2 was a 22-week trial consisting of a 3-week dose adjustment of current levodopa treatment prior to a 6-week conversion to carbidopa and levodopa extended-release capsules, which was followed by a 13-week, randomized, multicenter, double-blind, levodopa-containing active control, double-dummy, parallel group trial. The study enrolled 471 (393 randomized) patients (Hoehn & Yahr Stages I-IV) who had been maintained on a stable regimen of at least 400 mg per day of levodopa prior to entry into the trial. Patients were continued on concomitant dopamine agonists, selective monoamine oxidase B (MAO-B) inhibitors, amantadine, and anticholinergics provided the doses were stable for at least 4 weeks prior to screening. Patients were randomized to receive either carbidopa and levodopa extended-release capsules or immediate-release carbidopa-levodopa at the dose determined during the adjustment or conversion phases. Patients were not allowed to receive supplemental carbidopa-levodopa or catechol-O-methyl transferase (COMT) inhibitor products during the trial.
In Study 2, approximately 60% of patients required further up titration and approximately 16% of patients required down titration compared to the recommended starting dose of carbidopa and levodopa extended-release capsules. The final total daily dose of levodopa from carbidopa and levodopa extended-release capsules was approximately double that of the final total daily dose of levodopa from immediate-release tablets. The majority (88%) of patients in Study 2 received less than 2,400 mg; the median dose was 1,365 mg.
The clinical outcome measure in Study 2 was the percentage of "off" time during waking hours at Week 22 (or at early termination), as assessed by the patient's Parkinson's Disease Diary. The "off" time was significantly improved in carbidopa and levodopa extended-release capsules-treated patients compared to immediate-release carbidopa-levodopa-treated patients (Table 5). The decrease in "off" time observed with carbidopa and levodopa extended-release capsules occurred with a concomitant increase in "on time" without troublesome dyskinesia.
Table 5 Study 2: Parkinson's Disease Diary Measures in Patients with Advanced Parkinson's Disease - * P-value is less than 0.05
Baseline
Week 22
(or Early Termination)
Percentage of waking hours spent in "Off"
Carbidopa and Levodopa Extended-release Capsules
36.9%
23.8%*
Immediate-release carbidopa-levodopa
36%
29.8%
"Off" Time (hours)
Carbidopa and Levodopa Extended-release Capsules
6.1 hours
3.9 hours*
Immediate-release carbidopa-levodopa
5.9 hours
4.9 hours
"On" Time with no or non-troublesome
dyskinesia (hours)
Carbidopa and Levodopa Extended-release Capsules
10 hours
11.8 hours*
Immediate-release carbidopa-levodopa
10.1 hours
10.9 hours
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Carbidopa and Levodopa Extended-release Capsules are available in the following strengths:
● Carbidopa and Levodopa Extended-release Capsules, 23.75 mg/95 mg: blue and white capsule imprinted with IPX066 on the capsule cap and 95 on the capsule body and are supplied as follows:
Bottles of 100 NDC: 70710-2139-1
● Carbidopa and Levodopa Extended-release Capsules, 36.25 mg/145 mg: blue and light blue capsule imprinted with IPX066 on the capsule cap and 145 on the capsule body and are supplied as follows:
Bottles of 100 NDC: 70710-2140-1
● Carbidopa and Levodopa Extended-release Capsules, 48.75 mg/195 mg: blue and yellow capsule imprinted with IPX066 on the capsule cap and 195 on the capsule body and are supplied as follows:
Bottles of 100 NDC: 70710-2141-1
● Carbidopa and Levodopa Extended-release Capsules, 61.25 mg/245 mg: blue capsule imprinted with IPX066 on the capsule cap and 245 on the capsule body and are supplied as follows:
Bottles of 100 NDC: 70710-2142-1
16.2 Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Store in a tightly closed container, protected from light and moisture. Dispense in a tight, light-resistant container as defined in the USP.
-
17 PATIENT COUNSELING INFORMATION
- Advise patients to not take other carbidopa-levodopa preparations with carbidopa and levodopa extended-release capsules without consulting their healthcare provider [see Dosage and Administration (2.2)].
- Advise patients to call their healthcare provider before stopping carbidopa and levodopa extended-release capsules. Discontinue carbidopa and levodopa extended-release capsules slowly. Tell patients to call their healthcare provider if they develop withdrawal symptoms such as fever, confusion or severe muscle stiffness [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
- Advise patients to swallow carbidopa and levodopa extended-release capsules whole, without chewing, dividing, or crushing [see Dosage and Administration (2.4)].
- For patients with difficulty swallowing, the entire contents of the carbidopa and levodopa extended-release capsule may be sprinkled on 1 to 2 tablespoons of applesauce and should be taken immediately [see Dosage and Administration (2.4)].
- Inform patients that a high fat, high calorie meal may delay the absorption of levodopa and the onset of action by 2 to 3 hours. For this reason, consideration should be given to taking the first dose of the day about 1 to 2 hours before eating [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Advise patients that certain side effects such as sleepiness and dizziness that have been reported with carbidopa and levodopa extended-release capsules may affect some patients' ability to drive and operate machinery safely [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Suicide Attempt and Suicidal Ideation
Instruct patients, family members and caregivers to notify their healthcare provider if suicide attempt and/or suicidal ideation are experienced by patients using carbidopa and levodopa extended-release capsules [see Adverse Reactions (6.2)].
Hallucinations and Psychosis
Inform patients that hallucinations can occur with levodopa products [see Warnings and Precautions (5.4)].
Impulse Control Disorder
Inform patients of the potential for experiencing intense urges to gamble, increased sexual urges, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson's disease [see Warnings and Precautions (5.5)].
Dyskinesia
Instruct patients to notify their healthcare provider if abnormal involuntary movements appear or get worse during treatment with carbidopa and levodopa extended-release capsules [see Warnings and Precautions (5.6)].
Hypotension and Syncope
Advise patients that they may develop orthostatic hypotension with or without symptoms such as dizziness, nausea, syncope, and sweating [see Adverse Reactions (6.1)]. Advise patients to rise slowly after sitting or lying down, especially if they have been doing so for a prolonged period.
Advise patients of the possible additive sedative effects when taking other CNS depressants in combination with carbidopa and levodopa extended-release capsules.
Pregnancy and Breastfeeding
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during carbidopa and levodopa extended-release capsules therapy [see Use in Specific Populations (8.1)].
Advise female patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
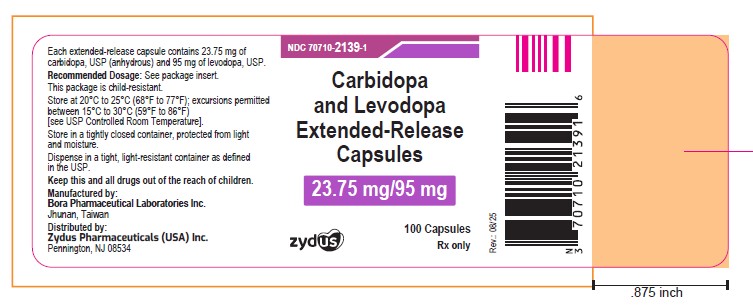
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 70710-2139-1
Carbidopa and Levodopa Extended-release Capsules, 23.75 mg/95 mg
Rx only
100 Capsules
ZYDUS
NDC: 70710-2140-1
Carbidopa and Levodopa Extended-release Capsules, 36.25 mg/145 mg
Rx only
100 Capsules
ZYDUS

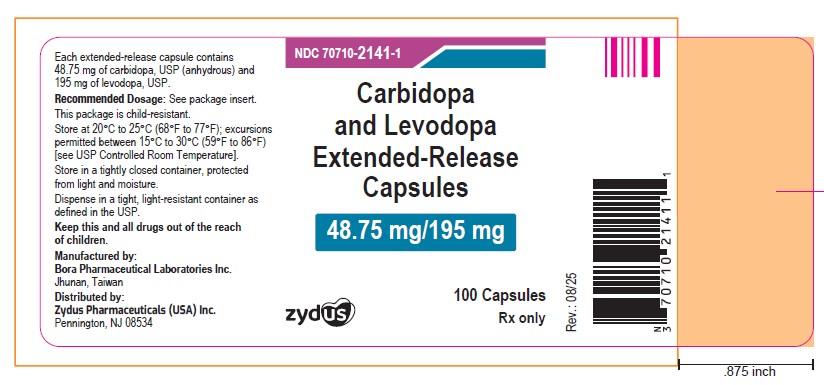
NDC: 70710-2141-1
Carbidopa and Levodopa Extended-release Capsules, 48.75 mg/195 mg
Rx only
100 Capsules
ZYDUS
NDC: 70710-2142-1
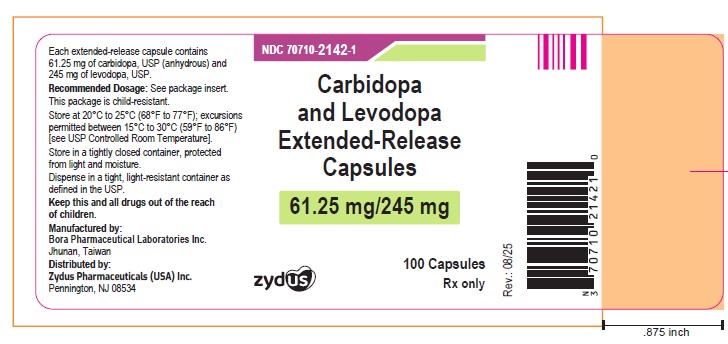
Carbidopa and Levodopa Extended-release Capsules, 61.25 mg/245 mg
Rx only
100 Capsules
ZYDUS
-
INGREDIENTS AND APPEARANCE
CARBIDOPA AND LEVODOPA
carbidopa and levodopa capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70710-2139 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBIDOPA (UNII: MNX7R8C5VO) (CARBIDOPA ANHYDROUS - UNII:KR87B45RGH) CARBIDOPA ANHYDROUS 23.75 mg LEVODOPA (UNII: 46627O600J) (LEVODOPA - UNII:46627O600J) LEVODOPA 95 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYLCELLULOSE (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color WHITE (WHITE) , BLUE (Blue) Score no score Shape CAPSULE (Oval) Size 18mm Flavor Imprint Code IPX066;95 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70710-2139-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/20/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA203312 10/20/2025 CARBIDOPA AND LEVODOPA
carbidopa and levodopa capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70710-2140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBIDOPA (UNII: MNX7R8C5VO) (CARBIDOPA ANHYDROUS - UNII:KR87B45RGH) CARBIDOPA ANHYDROUS 36.25 mg LEVODOPA (UNII: 46627O600J) (LEVODOPA - UNII:46627O600J) LEVODOPA 145 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYLCELLULOSE (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color BLUE (Blue) , BLUE (Light Blue) Score no score Shape CAPSULE (Oval) Size 19mm Flavor Imprint Code IPX066;145 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70710-2140-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/20/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA203312 10/20/2025 CARBIDOPA AND LEVODOPA
carbidopa and levodopa capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70710-2141 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBIDOPA (UNII: MNX7R8C5VO) (CARBIDOPA ANHYDROUS - UNII:KR87B45RGH) CARBIDOPA ANHYDROUS 48.75 mg LEVODOPA (UNII: 46627O600J) (LEVODOPA - UNII:46627O600J) LEVODOPA 195 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYLCELLULOSE (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color BLUE (Blue) , YELLOW (Yellow) Score no score Shape CAPSULE (Oval) Size 24mm Flavor Imprint Code IPX066;195 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70710-2141-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/20/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA203312 10/20/2025 CARBIDOPA AND LEVODOPA
carbidopa and levodopa capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70710-2142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBIDOPA (UNII: MNX7R8C5VO) (CARBIDOPA ANHYDROUS - UNII:KR87B45RGH) CARBIDOPA ANHYDROUS 61.25 mg LEVODOPA (UNII: 46627O600J) (LEVODOPA - UNII:46627O600J) LEVODOPA 245 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYLCELLULOSE (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color BLUE (Blue) , BLUE (Blue) Score no score Shape CAPSULE (Oval) Size 23mm Flavor Imprint Code IPX066;245 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70710-2142-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/20/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA203312 10/20/2025 Labeler - Zydus Pharmaceuticals USA Inc. (156861945) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals Pvt. Ltd. 915076126 ANALYSIS(70710-2139, 70710-2140, 70710-2141, 70710-2142) , LABEL(70710-2139, 70710-2140, 70710-2141, 70710-2142) , MANUFACTURE(70710-2139, 70710-2140, 70710-2141, 70710-2142) , PACK(70710-2139, 70710-2140, 70710-2141, 70710-2142) Establishment Name Address ID/FEI Business Operations Bora Pharmaceutical Laboratories Inc 656139511 ANALYSIS(70710-2139, 70710-2140, 70710-2141, 70710-2142) , LABEL(70710-2139, 70710-2140, 70710-2141, 70710-2142) , MANUFACTURE(70710-2139, 70710-2140, 70710-2141, 70710-2142) , PACK(70710-2139, 70710-2140, 70710-2141, 70710-2142)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.