SENNA-LAX- sennosides tablet
Senna-Lax by
Drug Labeling and Warnings
Senna-Lax by is a Otc medication manufactured, distributed, or labeled by L&R Distributors, Inc., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
-
Directions
- take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children 12 years and over 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
select brand®
the lower price name brandNDC: 15127-105-01
Natural Vegetable
LaxativeSenna-Lax
Sennosides USP, 8.6 mg- LaxativeGently Relieves Constipation
Actual
Size*Compare to the active ingredient in Senokot®
100 Tablets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed by
Purdue Products L.P., owner of the registered
trademark Senokot®.
50844 REV1216C29812Distributed by:
SELECT BRAND® DISTRIBUTORS
Pine Bluff, AR 71603 USA
AC (870) 535-3635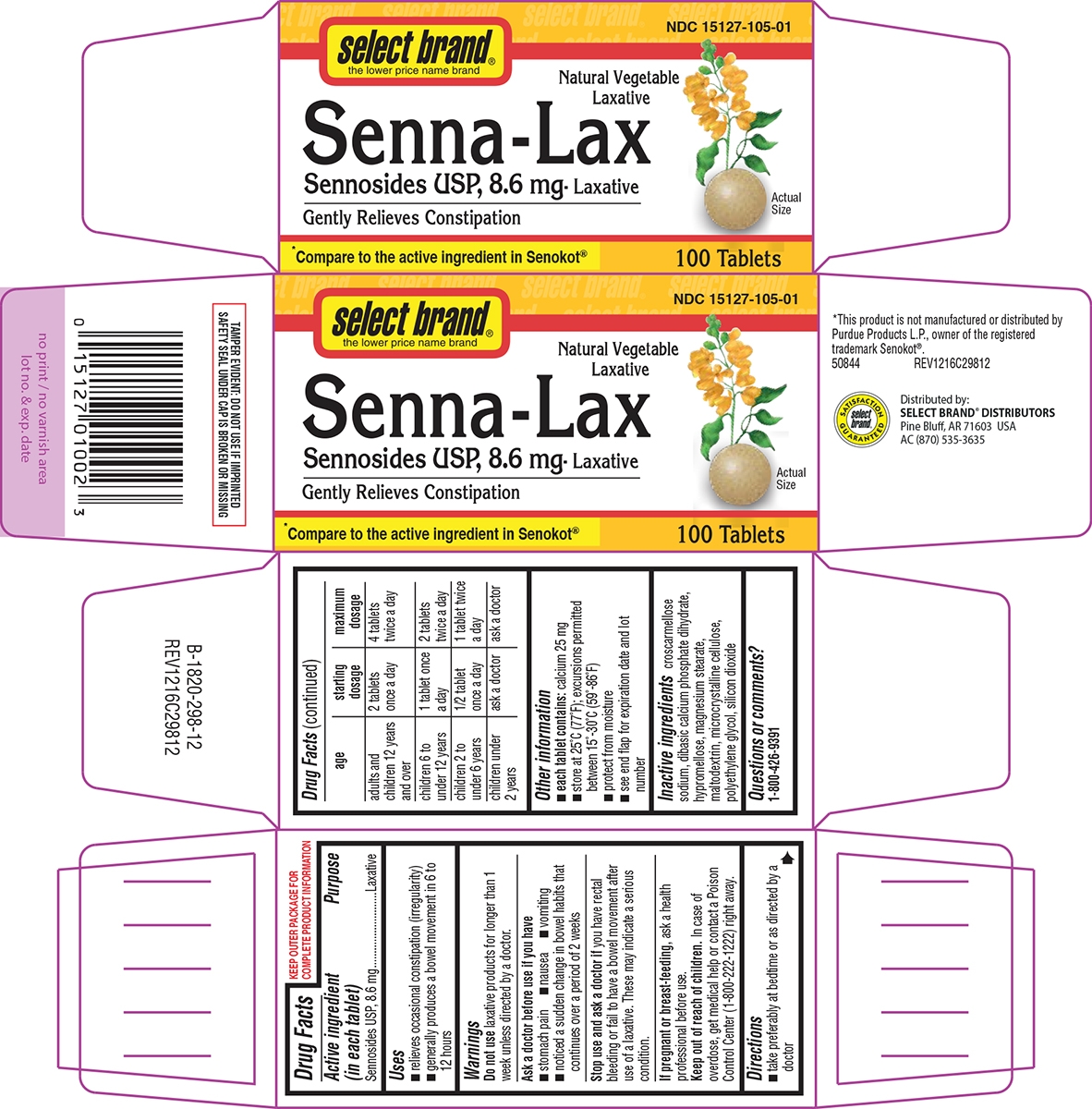
Select Brand 44-298
-
INGREDIENTS AND APPEARANCE
SENNA-LAX
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 15127-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Product Characteristics Color BROWN (greenish) Score no score Shape ROUND Size 9mm Flavor Imprint Code 44;298 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15127-105-01 1 in 1 CARTON 01/27/1995 09/10/2021 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 01/27/1995 09/10/2021 Labeler - L&R Distributors, Inc. (012578514) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(15127-105) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(15127-105) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(15127-105) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(15127-105)
Trademark Results [Senna-Lax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENNA-LAX 73470768 1460560 Live/Registered |
Nature's Herbs, Inc. 1984-03-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.