DEXTENZA- dexamethasone insert
DEXTENZA by
Drug Labeling and Warnings
DEXTENZA by is a Prescription medication manufactured, distributed, or labeled by Ocular Therapeutix, Inc., Isomedix Operations, Steris Corporation, Packaging Coordinators, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA.
DEXTENZA® (dexamethasone ophthalmic insert) 0.4 mg, for intracanalicular use
Initial U.S. Approval: 1958RECENT MAJOR CHANGES
Indications and Usage (1) 06/2019 INDICATIONS AND USAGE
DEXTENZA® is a corticosteroid indicated for the treatment of ocular inflammation and pain following ophthalmic surgery (1).
DOSAGE AND ADMINISTRATION
DEXTENZA is an ophthalmic insert that is inserted in the lower lacrimal punctum and into the canaliculus. A single DEXTENZA releases a 0.4 mg dose of dexamethasone for up to 30 days following insertion (2).
DOSAGE FORMS AND STRENGTHS
Ophthalmic intracanalicular insert containing a 0.4 mg dose of dexamethasone (3).
CONTRAINDICATIONS
Active ocular infections (4).
WARNINGS AND PRECAUTIONS
- Intraocular Pressure Increase: Monitor intraocular pressure (5.1).
- Bacterial Infections: Steroids may mask signs of infections and enhance existing infections (5.2).
- Viral Infections: Ocular steroids may prolong the course and exacerbate the severity of ocular viral infections (5.3).
- Fungal Infections: Consider fungal invasion in any persistent corneal ulceration (5.4).
- Delayed Healing: Ocular steroids may slow the rate of ocular healing. (5.5).
ADVERSE REACTIONS
The most commonly reported adverse reactions were anterior chamber inflammation and elevations in intraocular pressure. These occurred in approximately 6-10% of patients (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Ocular Therapeutix at 1-800-DEXTENZA (339-8369) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Intraocular Pressure Increase
5.2 Bacterial Infection
5.3 Viral Infections
5.4 Fungal Infections
5.5 Delayed Healing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
DEXTENZA® (dexamethasone ophthalmic insert) is a corticosteroid indicated for the treatment of ocular inflammation and pain following ophthalmic surgery (1).
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
DEXTENZA is an ophthalmic insert that is inserted in the lower lacrimal punctum into the canaliculus. A single DEXTENZA insert releases a 0.4 mg dose of dexamethasone for up to 30 days following insertion.
DEXTENZA is resorbable and does not require removal. Saline irrigation or manual expression can be performed to remove the insert if necessary. DEXTENZA is intended for single-use only.
2.2 Administration
Do not use if pouch has been damaged or opened. Do not re-sterilize.
- Carefully remove foam carrier and transfer to a clean and dry area.
- If necessary, dilate the punctum with an ophthalmic dilator. Care should be taken not to perforate the canaliculus during dilation or insertion of DEXTENZA. If perforation occurs, do not insert DEXTENZA.
- After drying the punctal area, using blunt (non-toothed) forceps, grasp DEXTENZA and insert into the lower lacrimal canaliculus. DEXTENZA should be placed just below the punctal opening. Excessive squeezing of DEXTENZA may cause deformation.
- To aid in the hydration of DEXTENZA, 1 to 2 drops of balanced salt solution can be instilled into the punctum. DEXTENZA hydrates quickly upon contact with moisture. If DEXTENZA begins to hydrate before fully inserted, discard the product and use a new DEXTENZA.
- DEXTENZA can be visualized when illuminated by a blue light source (e.g., slit lamp or hand held blue light) with yellow filter.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Intraocular Pressure Increase
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be monitored during the course of the treatment.
5.2 Bacterial Infection
Corticosteroids may suppress the host response and thus increase the hazard for secondary ocular infections. In acute purulent conditions, steroids may mask infection and enhance existing infection [see Contraindications (4)].
5.3 Viral Infections
Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex) [see Contraindications (4)].
5.4 Fungal Infections
Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use. Fungal culture should be taken when appropriate [see Contraindications (4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Intraocular Pressure Increase [see Warnings and Precautions (5.1)]
- Bacterial Infection [see Warnings and Precautions (5.2)]
- Viral Infection [see Warnings and Precautions (5.3)]
- Fungal Infection [see Warnings and Precautions (5.4)]
- Delayed Healing [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Adverse reactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation; delayed wound healing; secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera [see Warnings and Precautions (5)].
DEXTENZA was studied in four randomized, vehicle-controlled studies (n = 567). The mean age of the population was 68 years (range 35 to 87 years), 59% were female, and 83% were white. Forty-seven percent had brown iris color and 30% had blue iris color. The most common ocular adverse reactions that occurred in patients treated with DEXTENZA were: anterior chamber inflammation including iritis and iridocyclitis (10%); intraocular pressure increased (6%); visual acuity reduced (2%); cystoid macular edema (1%); corneal edema (1%); eye pain (1%) and conjunctival hyperemia (1%).
The most common non-ocular adverse reaction that occurred in patients treated with DEXTENZA was headache (1%).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate or well-controlled studies with DEXTENZA in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. In animal reproduction studies, administration of topical ocular dexamethasone to pregnant mice and rabbits during organogenesis produced embryofetal lethality, cleft palate and multiple visceral malformations [see Animal Data].
Data
Animal Data
Topical ocular administration of 0.15% dexamethasone (0.75 mg/kg/day) on gestational days 10 to 13 produced embryofetal lethality and a high incidence of cleft palate in a mouse study. A daily dose of 0.75 mg/kg/day in the mouse is approximately 5 times the entire dose of dexamethasone in the DEXTENZA product, on a mg/m2 basis. In a rabbit study, topical ocular administration of 0.1% dexamethasone throughout organogenesis (0.36 mg /day, on gestational day 6 followed by 0.24 mg/day on gestational days 7-18) produced intestinal anomalies, intestinal aplasia, gastroschisis and hypoplastic kidneys. A daily dose of 0.24 mg/day is approximately 6 times the entire dose of dexamethasone in the DEXTENZA product, on a mg/m2 basis.
8.2 Lactation
Systemically administered corticosteroids appear in human milk and could suppress growth and interfere with endogenous corticosteroid production; however the systemic concentration of dexamethasone following administration of DEXTENZA is low [see Clinical Pharmacology (12.3)]. There is no information regarding the presence of DEXTENZA in human milk, the effects of the drug on the breastfed infant or the effects of the drug on milk production to inform risk of DEXTENZA to an infant during lactation. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DEXTENZA and any potential adverse effects on the breastfed child from DEXTENZA.
-
11 DESCRIPTION
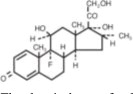
DEXTENZA (dexamethasone ophthalmic insert) is a fluorescent yellow, 3 mm cylindrical-shaped, resorbable, sterile insert for intracanalicular use. DEXTENZA contains 0.4 mg dexamethasone in a polyethylene glycol (PEG) based hydrogel conjugated with fluorescein. DEXTENZA does not contain an antimicrobial preservative. The active ingredient is represented by the chemical structure:
The chemical name for dexamethasone is 9-Fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione. It has a molecular formula of C22H29FO5 and a molecular weight of 392.47 g/mol. Dexamethasone is a crystalline powder.
Each DEXTENZA contains: Active ingredients: 0.4 mg dexamethasone. Inactive ingredients: 4-arm polyethylene glycol (PEG) N-hydroxysuccinimidyl glutarate (20K), trilysine acetate, N-hydroxysuccinimide-fluorescein, sodium phosphate dibasic, sodium phosphate monobasic, water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dexamethasone, a corticosteroid, has been shown to suppress inflammation by inhibiting multiple inflammatory cytokines resulting in decreased edema, fibrin deposition, capillary leakage and migration of inflammatory cells.
12.3 Pharmacokinetics
Plasma samples were obtained from 16 healthy volunteers prior to insertion of DEXTENZA and on Day 1 (at 1, 2, 4, 8, 16 hours), 2 (24 hours), 4, 8, 15, 22 and 29 following the insertion of DEXTENZA.
Plasma concentrations of dexamethasone were detectable (above 50 pg/mL, the lower limit of quantification of the assay) in 11% of samples (21 of 189), and ranged from 0.05 ng/mL to 0.81 ng/mL.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies in animals have been conducted to determine whether DEXTENZA has the potential for carcinogenesis.
Dexamethasone was not mutagenic in the Ames/Salmonella assay, both with and without metabolic activation. Dexamethasone was genotoxic in two in vitro assays using human lymphocytes (chromosomal aberration assay and sister chromatid exchange assay) and was genotoxic in two mouse in vivo assays (micronucleus assay and sister chromatid exchange assay).
Fertility studies have not been conducted in animals using DEXTENZA.
-
14 CLINICAL STUDIES
In three randomized, multicenter, double-masked, parallel group, vehicle-controlled trials, patients received DEXTENZA or its vehicle immediately upon completion of cataract surgery. In all three trials, DEXTENZA had a higher proportion of patients than the vehicle group who were pain free on post-operative Day 8. On post-operative Day 14, in two of the three studies, DEXTENZA had a higher proportion of patients than the vehicle group who had an absence of anterior chamber cells that was statistically significant. Results are shown in Table 1 and Table 2.
Table 1: Percentage of Patients with Absence of Anterior Chamber Cells Study 1 Study 2 Study 3 DEXTENZA
(N=164)Vehicle
(N=83)Difference
(95% CI)DEXTENZA
(N=161)Vehicle
(N=80)Difference
(95% CI)DEXTENZA
(N=216)Vehicle
(N=222)Difference
(95% CI)Visit n (%) n (%) n (%) n (%) n (%) n (%) Day 14 54 (33%) 12 (14%) 18% (8%, 29%) 63 (39%) 25 (31%) 8% (-5%, 21%) 113 (52%) 69 (31%) 21 % (12%, 30%) Table 2: Percentage of Patients with Absence of Pain Study 1 Study 2 Study 3 DEXTENZA
(N=164)Vehicle
(N=83)Difference
(95% CI)DEXTENZA
(N=161)Vehicle
(N=80)Difference
(95% CI)DEXTENZA
(N=216)Vehicle
(N=222)Difference
(95% CI)Visit n (%) n (%) n (%) n (%) n (%) n (%) Day 8 131 (80%) 36 (43%) 37% (24%, 49%) 124 (77%) 47 (59%) 18% (6%, 31%) 172 (80%) 136 (61%) 18% (10%, 27%) -
16 HOW SUPPLIED/STORAGE AND HANDLING
DEXTENZA is supplied sterile in a foam carrier within a foil laminate pouch containing:
NDC: 70382-204-10 Carton containing 10 pouches (10 inserts) NDC: 70382-204-01 Carton containing 1 pouch (1 insert) Do not use if pouch has been damaged or broken.
DEXTENZA is intended for single dose only.
- 17 PATIENT COUNSELING INFORMATION
-
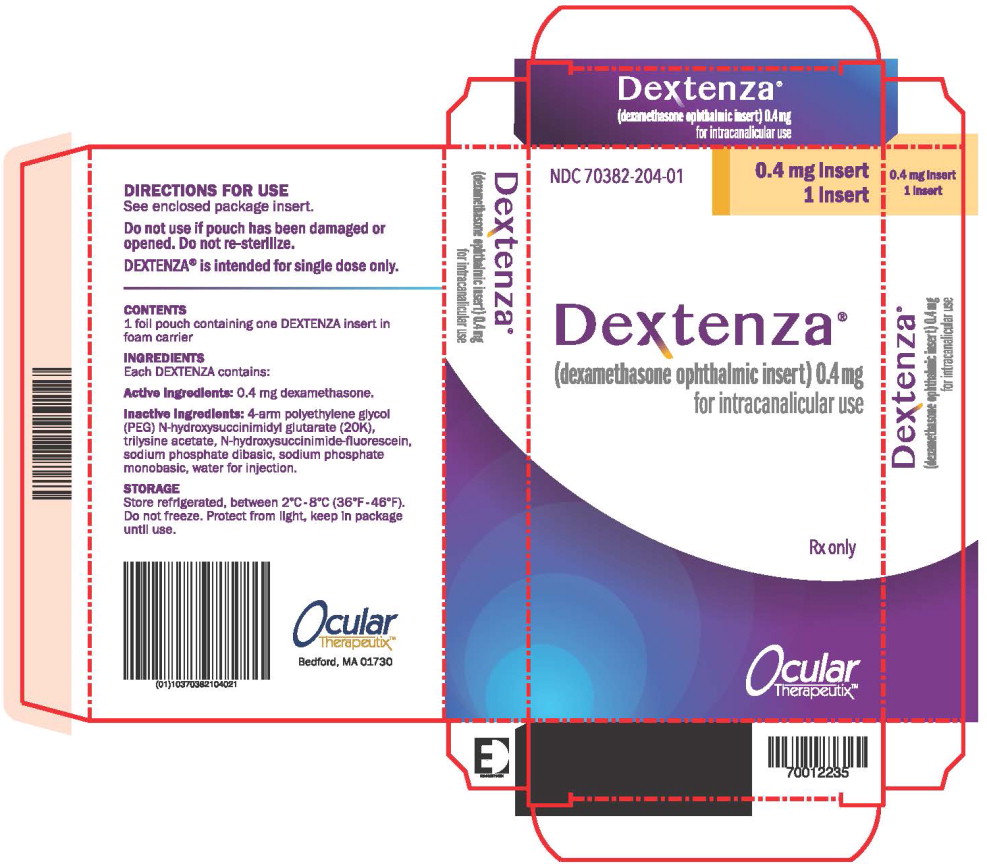
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Dextenza 1 ct Box Label
NDC: 70382-204-01
0.4 mg insert
1 insert
Dextenza®
(dexamethasone ophthalmic insert) 0.4mg
for intracanalicular use
Rx only
Ocular
Therapeutix™
-
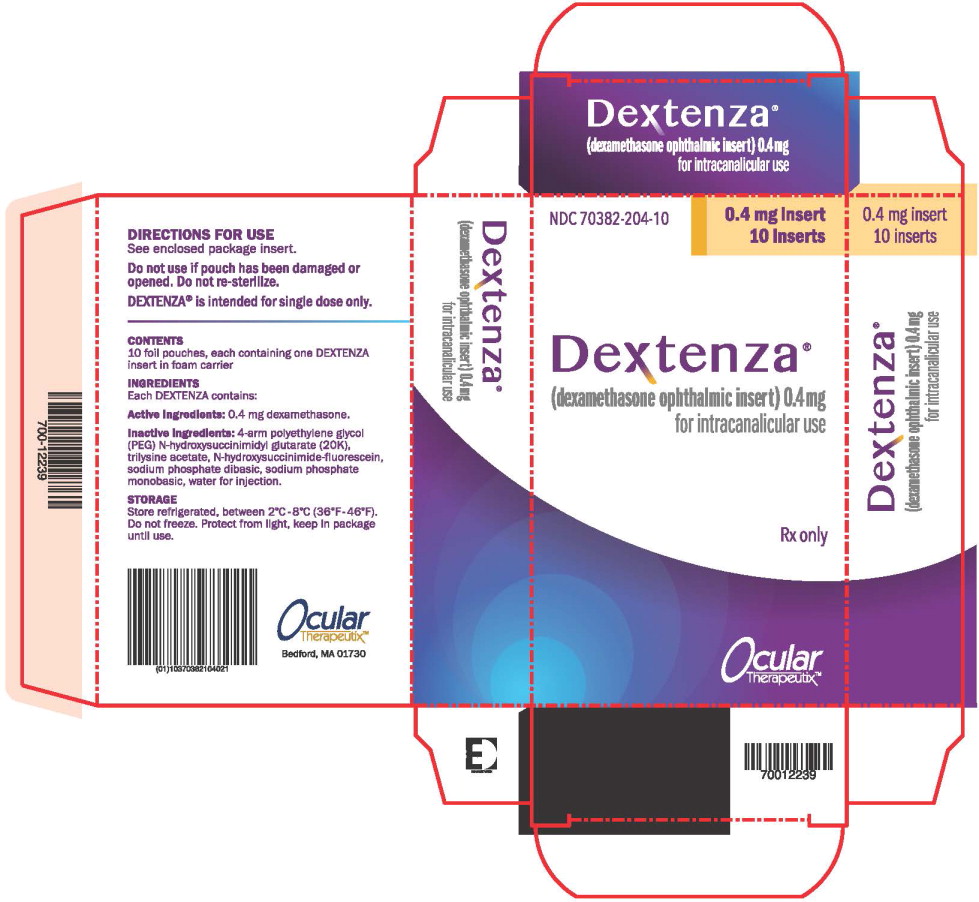
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Dextenza 10 ct Box Label
NDC: 70382-204-10
0.4 mg insert
10 inserts
Dextenza®
(dexamethasone ophthalmic insert) 0.4mg
for intracanalicular use
Rx only
Ocular
Therapeutix™
-
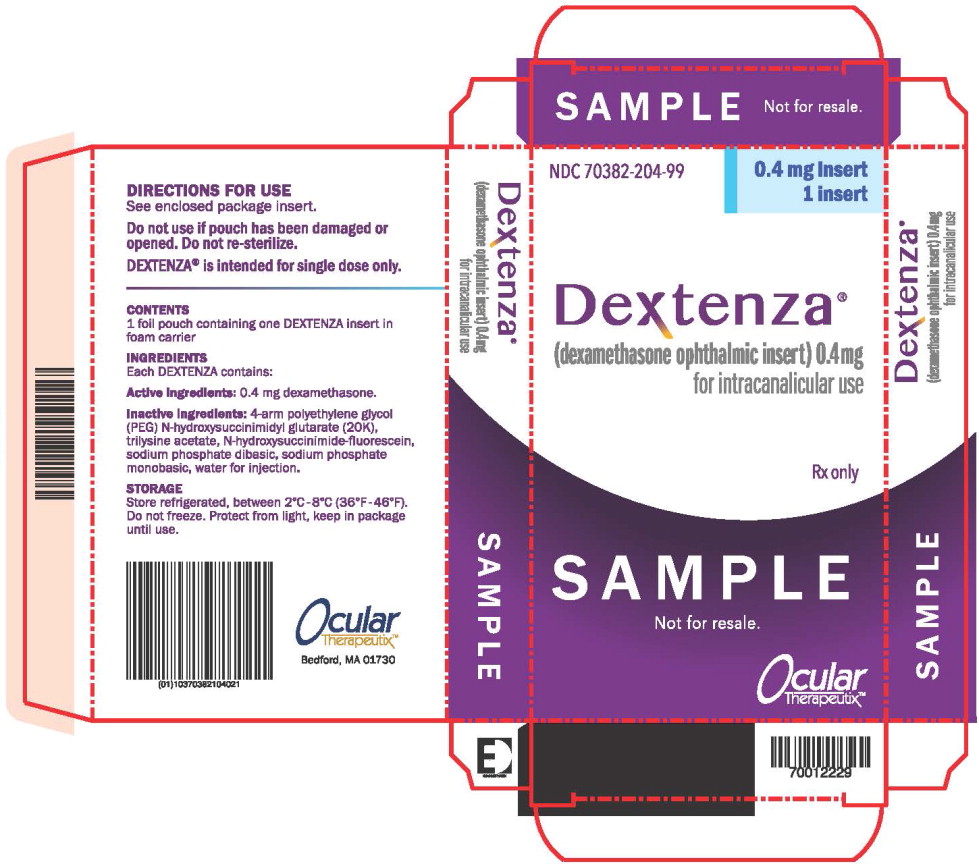
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Dextenza Sample 1 ct Box Label
NDC: 70382-204-99
0.4 mg insert
1 inserts
Dextenza®
(dexamethasone ophthalmic insert) 0.4mg
for intracanalicular use
Rx only
SAMPLE
Not for resale.
US Patent Nos.
7,648,713 8,409,606 8,563,027 9,254,267
Ocular
Therapeutix™
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Dextenza Pouch Label
Dextenza®
(dexamethasone ophthalmic insert) 0.4mg
for intracanalicular use
Ocular Therapeutix, Inc.
Bedford, MA 01730 USA
NDC: 70382-204-88
Rx only
LOT:
EXP DATE:
DIRECTIONS FOR USE: See enclosed package
insert. Do not use if pouch has been damaged
or broken. DEXTENZA is intended for single dose only.CONTENTS: One DEXTENZA insert in foam carrier.
STORAGE: Refrigerate between 2 ° C - 8 ° C
(36° F - 46° F). Do not freeze. Protect from light,
keep in package until use.STERILE: Do not re-sterilize.
PCR-780-12173
-
INGREDIENTS AND APPEARANCE
DEXTENZA
dexamethasone insertProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70382-204 Route of Administration OPHTHALMIC, INTRACANALICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength dexamethasone (UNII: 7S5I7G3JQL) (dexamethasone - UNII:7S5I7G3JQL) dexamethasone 0.4 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) LYSYLLYSYLLYSINE (UNII: Y7F82M80K8) FLUORESCEIN (UNII: TPY09G7XIR) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) Product Characteristics Color yellow (yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70382-204-01 1 in 1 BOX, UNIT-DOSE 01/07/2019 1 NDC: 70382-204-88 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC: 70382-204-10 10 in 1 BOX, UNIT-DOSE 01/07/2019 2 NDC: 70382-204-88 1 in 1 POUCH; Type 0: Not a Combination Product 3 NDC: 70382-204-99 1 in 1 BOX, UNIT-DOSE 01/07/2019 3 NDC: 70382-204-88 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208742 01/07/2019 Labeler - Ocular Therapeutix, Inc. (063391985) Establishment Name Address ID/FEI Business Operations Isomedix Operations, Steris Corporation 117383794 STERILIZE(70382-204) Establishment Name Address ID/FEI Business Operations Ocular Therapeutix, Inc. 080122594 MANUFACTURE(70382-204) Establishment Name Address ID/FEI Business Operations Packaging Coordinators, Inc. 078525133 LABEL(70382-204) , PACK(70382-204)
Trademark Results [DEXTENZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DEXTENZA 87864954 not registered Live/Pending |
Ocular Therapeutix, Inc. 2018-04-05 |
 DEXTENZA 86425297 5513989 Live/Registered |
Ocular Therapeutix, Inc. 2014-10-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.