SELASPOT- selamectin solution
SelaSpot by
Drug Labeling and Warnings
SelaSpot by is a Animal medication manufactured, distributed, or labeled by Vedco, Inc., Bimeda, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION:
SelaSpot (selamectin) Topical Parasiticide is available as a colorless to yellow, ready to use solution in single dose tubes for topical (dermal) treatment of dogs six weeks of age and older and cats eight weeks of age and older. The content of each tube is formulated to provide a minimum of 2.7 mg/lb (6 mg/kg) of body weight of selamectin. The chemical composition of selamectin is (5Z,25S)-25-cyclohexyl-4'-O-de(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-5-demethoxy-25-de(1-methylpropyl)-22,23-dihydro-5-hydroxyiminoavermectin A1a. -
INDICATIONS & USAGE
INDICATIONS:
SelaSpot is recommended for use in dogs six weeks of age or older and cats eight weeks of age and older for the following parasites and indications:Dogs:
SelaSpot kills adult fleas and prevents flea eggs from hatching for one month and is indicated for the prevention and control of flea infestations (Ctenocephalides felis), prevention of heartworm disease caused by Dirofilaria immitis, and the treatment and control of ear mite (Otodectes cynotis) infestations. SelaSpot also is indicated for the treatment and control of sarcoptic mange (Sarcoptes scabiei) and for the control of tick infestations due to Dermacentor variabilis.Cats:
SelaSpot kills adult fleas and prevents flea eggs from hatching for one month and is indicated for the prevention and control of flea infestations (Ctenocephalides felis), prevention of heartworm disease caused by Dirofilaria immitis, and the treatment and control of ear mite (Otodectes cynotis) infestations. SelaSpot is also indicated for the treatment and control of roundworm (Toxocara cati) and intestinal hookworm (Ancylostoma tubaeforme) infections in cats. -
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
The recommended minimum dose is 2.7 mg selamectin per pound (6 mg/kg) of body weight.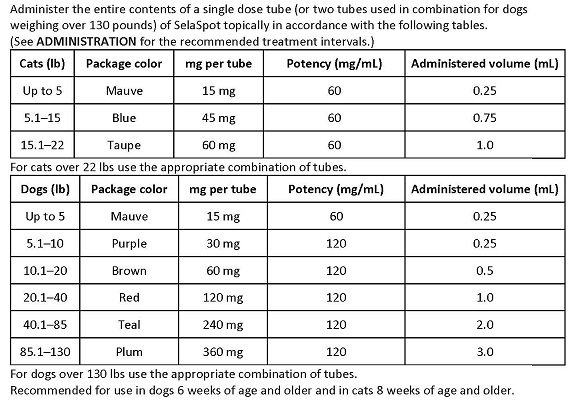
A veterinarian or veterinary technician should demonstrate or instruct the pet owner regarding the appropriate technique for applying SelaSpot topically to dogs and cats prior to first use.
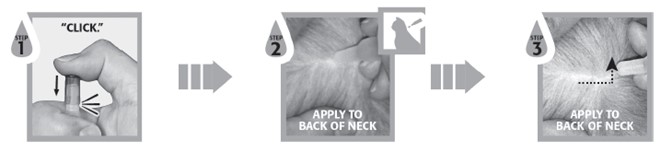
Firmly press the cap down to puncture the seal on the SelaSpot tube; a clicking sound will confirm that the cap has successfully punctured the seal. Remove the cap and check to ensure that the tip of the tube is open. To administer the product, part the hair on the back of the animal at the base of the neck in front of the shoulder blades until the skin is visible. Place the tip of the tube on the skin and squeeze the tube 3 or 4 times to empty its entire contents directly onto the skin in one spot. Keeping the tube squeezed, drag it away from the liquid and lift to remove. Check the tube to ensure that it is empty. Do not massage the product into the skin. Due to alcohol content, do not apply to broken skin. Avoid contact between the product and fingers. Do not apply when the haircoat is wet. Bathing or shampooing the dog 2 or more hours after treatment will not reduce the effectiveness of SelaSpot against fleas or heartworm. Bathing or shampooing the cat 2 hours after treatment will not reduce the effectiveness of SelaSpot against fleas. Bathing or shampooing the cat 24 hours after treatment will not reduce the effectiveness of SelaSpot against heartworm. Stiff hair, clumping of hair, hair discoloration, or a slight powdery residue may be observed at the treatment site in some animals. These effects are temporary and do not affect the safety or effectiveness of the product. Discard empty tubes in your ordinary household refuse.
Flea Control in Dogs and Cats
For the prevention and control of flea infestations, SelaSpot should be administered at monthly intervals throughout the flea season, starting one month before fleas become active. In controlled laboratory studies >98% of fleas were killed within 36 hours. Results of clinical field studies using selamectin monthly demonstrated >90% control of flea infestations within 30 days of the first dose. Dogs and cats treated with selamectin, including those with pre-existing flea allergy dermatitis, showed improvement in clinical signs associated with fleas as a direct result of eliminating the fleas from the animals and their environment.
If the dog or cat is already infested with fleas when the first dose of SelaSpot is administered, adult fleas on the animal are killed and no viable fleas hatch from eggs after the first administration. However, an environmental infestation of fleas may persist for a short time after beginning treatment with SelaSpot because of the emergence of adult fleas from pupae.
Heartworm Prevention in Dogs and Cats
For the prevention of heartworm disease, SelaSpot must be administered on a monthly basis. SelaSpot may be administered year-round or at least within one month after the animal's first exposure to mosquitoes and monthly thereafter until the end of the mosquito season. The final dose must be given within one month after the last exposure to mosquitoes. If a dose is missed and a monthly interval between dosing is exceeded then immediate administration of SelaSpot and resumption of monthly dosing will minimize the opportunity for the development of adult heartworms. When replacing another heartworm preventive product in a heartworm disease prevention program, the first dose of SelaSpot must be given within a month of the last dose of the former medication.Selamectin, the active ingredient in SelaSpot, is a macrocyclic lactone compound. These compounds effectively prevent the development of adult heartworms when administered to dogs and cats within one month of exposure to infective (L3) Dirofilaria immitis larvae. Efficacy of macrocyclic lactones decreases below 100% in dogs, however, if first administered >2 months after exposure to infective larvae. Thus, in heartworm endemic regions, delaying initiation of heartworm prevention using SelaSpot beyond 2 months of first exposure to infective larvae (e.g., starting puppies and kittens at >8 weeks of age), or gaps of >2 months in the administration of SelaSpot during periods of heartworm transmission, increases the risk of the animal acquiring heartworms. Animals with unknown heartworm history that test negative for heartworms prior to the initiation of SelaSpot may be harboring pre-patent infections at the time SelaSpot was started. Testing such animals 3–4 months after initiation of SelaSpot would be necessary to confirm their negative heartworm status.
At the discretion of the veterinarian, cats ≥6 months of age may be tested to determine the presence of existing heartworm infections before beginning treatment with SelaSpot. Cats already infected with adult heartworms can be given SelaSpot monthly to prevent further infections.
Ear Mite Treatment in Dogs and Cats
For the treatment of ear mite (O. cynotis) infestations in dogs and cats, SelaSpot should be administered once as a single topical dose. A second monthly dose may be required in some dogs. Monthly use of SelaSpot will control any subsequent ear mite infestations. In the clinical field trials ears were not cleaned, and many animals still had debris in their ears after the second dose. Cleansing of the infested ears is recommended to remove the debris.Sarcoptic Mange Treatment in Dogs
For the treatment of sarcoptic mange (S. scabiei) in dogs, SelaSpot should be administered once as a single topical dose. A second monthly dose may be required in some dogs. Monthly use of SelaSpot will control any subsequent sarcoptic mange mite infestations. Because of the difficulty in finding sarcoptic mange mites on skin scrapings, effectiveness assessments also were based on resolution of clinical signs. Resolution of the pruritus associated with the mite infestations was observed in approximately 50% of the dogs 30 days after the first treatment and in approximately 90% of the dogs 30 days after the second monthly treatment.Tick Control in Dogs
For the control of tick (Dermacentor variabilis) infestations in dogs, SelaSpot should be administered on a monthly basis. In heavy tick infestations, complete efficacy may not be achieved after the first dose. In these cases, one additional dose may be administered two weeks after the previous dose, with monthly dosing continued thereafter.Nematode Treatment in Cats
For the treatment and control of intestinal hookworm (A. tubaeforme) and roundworm (T. cati) infections, SelaSpot should be applied once as a single topical dose. -
WARNINGS
WARNINGS:
User Safety Warnings
Not for human use. Keep out of the reach of children.
In humans, SelaSpot may be irritating to skin and eyes.
Reactions such as hives, itching and skin redness have been reported in humans. Individuals with known hypersensitivity to SelaSpot should use the product with caution or consult a health care professional. SelaSpot contains isopropyl alcohol and the preservative butylated hydroxytoluene (BHT). Wash hands after use and wash off any product in contact with the skin immediately with soap and water. If contact with eyes occurs, then flush eyes copiously with water; if wearing contact lenses, rinse the eyes first then remove contact lenses and continue to rinse for 5-10 minutes and seek medical attention.
In case of ingestion by a human, contact a physician immediately.
The safety data sheet (SDS) provides more detailed occupational safety information. To obtain a SDS contact Bimeda, Inc. at 1-888-524-6332 or www.bimeda.com.Flammable - Keep away from heat, sparks, open flames or other sources of ignition.
Animal Safety Warnings
Do not use in sick, debilitated or underweight animals (see TARGET ANIMAL SAFETY). -
PRECAUTIONS
PRECAUTIONS:
Prior to administration of SelaSpot, dogs should be tested for existing heartworm infections. At the discretion of the veterinarian, infected dogs should be treated to remove adult heartworms. SelaSpot is not effective against adult D. immitis and, while the number of circulating microfilariae may decrease following treatment, SelaSpot is not effective for microfilariae clearance.Hypersensitivity reactions have not been observed in dogs with patent heartworm infections administered selamectin (see TARGET ANIMAL SAFETY).
-
ADVERSE REACTIONS
ADVERSE REACTIONS:
Pre-Approval Clinical Trials:
Following treatment with selamectin, transient localized alopecia with or without inflammation at or near the site of application was observed in approximately 1% of 691 treated cats. Other signs observed (≤0.5% of 1743 treated cats and dogs) included vomiting, loose stool or diarrhea with or without blood, anorexia, lethargy, salivation, tachypnea, and muscle tremors.
Post-Approval Experience (2021):
The following adverse events are based on post-approval adverse drug experience reporting for selamectin. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data.
The following adverse events reported for dogs are listed in decreasing order of reporting frequency:
Lethargy, vomiting, diarrhea, anorexia, generalized pruritus, seizures, application site reactions (including alopecia, lesions, erythema, pruritis, and inflammation), tremors, ataxia, death, and dermatitis.The following adverse events reported for cats are listed in decreasing order of reporting frequency:
Application site reactions (including alopecia, lesions, erythema, pruritis, inflammation, vesicles, blisters, and excoriations), lethargy, anorexia, vomiting, death, generalized pruritis, diarrhea, ataxia, fever, generalized alopecia, tremors, hypersalivation, dermatitis, and seizures.CONTACT INFORMATION:
Contact Bimeda, Inc. at 1-888-524-6332 or www.bimeda.com. To report suspected drug experiences, contact Bimeda, Inc. at 1-888-534-6332. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae. -
OTHER SAFETY INFORMATION
TARGET ANIMAL SAFETY:
Selamectin has been tested safe in over 100 different pure and mixed breeds of healthy dogs and over 15 different pure and mixed breeds of healthy cats, including pregnant and lactating females, breeding males and females, puppies six weeks of age and older, kittens eight weeks of age and older, and avermectin-sensitive collies. A kitten, estimated to be 5–6 weeks old (0.3 kg), died 8 1⁄2 hours after receiving a single treatment of selamectin at the recommended dosage. The kitten displayed clinical signs which included muscle spasms, salivation and neurological signs. The kitten was a stray with an unknown history and was malnourished and underweight (see WARNINGS).
DOGS: In safety studies, selamectin was administered at 1, 3, 5, and 10 times the recommended dose to six-week-old puppies, and no adverse reactions were observed. The safety of selamectin administered orally also was tested in case of accidental oral ingestion. Oral administration of selamectin at the recommended topical dose in 5- to 8-month-old beagles did not cause any adverse reactions. In a pre-clinical study selamectin was dosed orally to ivermectin-sensitive collies. Oral administration of 2.5, 10, and 15 mg/kg in this dose escalating study did not cause any adverse reactions; however, eight hours after receiving 5 mg/kg orally, one avermectin-sensitive collie became ataxic for several hours, but did not show any other adverse reactions after receiving subsequent doses of 10 and 15 mg/kg orally. In a topical safety study conducted with avermectin-sensitive collies at 1, 3 and 5 times the recommended dose of selamectin, salivation was observed in all treatment groups, including the vehicle control. Selamectin also was administered at 3 times the recommended dose to heartworm infected dogs, and no adverse effects were observed.
CATS: In safety studies, selamectin was applied at 1, 3, 5, and 10 times the recommended dose to six-week-old kittens. No adverse reactions were observed. The safety of selamectin administered orally also was tested in case of accidental oral ingestion. Oral administration of the recommended topical dose of selamectin to cats caused salivation and intermittent vomiting. Selamectin also was applied at 4 times the recommended dose to patent heartworm infected cats, and no adverse reactions were observed.
In well-controlled clinical studies, selamectin was used safely in animals receiving other frequently used veterinary products such as vaccines, anthelmintics, antiparasitics, antibiotics, steroids, collars, shampoos and dips. - STORAGE AND HANDLING
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
INFORMATION FOR OWNERS/CAREGIVERS
Client Information About
SelaSpotTM (selamectin)
SelaSpot (pronounced “Sel-a-Spot”)
Generic name: selamectin ("sel-a-mec-tin")This summary contains important information about SelaSpot. You should read this information before you start using SelaSpot on your dog or cat and review it each time your prescription is refilled. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or if you want to know more about SelaSpot.
What is SelaSpot?
SelaSpot is a topical parasiticide that is applied to the skin of dogs six weeks of age and older and cats eight weeks of age and older to kill adult fleas and prevent flea eggs from hatching, prevent heartworm disease and protect your pet against other parasites (see below).Why has my veterinarian prescribed SelaSpot?
SelaSpot has been prescribed by your veterinarian to treat, prevent and/or control the following parasites in your dog or cat:Dog Parasites:
- Control and prevention of flea infestation (Ctenocephalides felis)
- Prevention of heartworm disease (Dirofilaria immitis)
- Treatment and control of ear mite infestation (Otodectes cynotis)
- Treatment and control of sarcoptic mange (Sarcoptes scabiei)
- Control of the American Dog Tick (Dermacentor variabilis)
Cat Parasites:
- Control and prevention of flea infestation (Ctenocephalides felis)
- Prevention of heartworm disease (Dirofilaria immitis)
- Treatment and control of ear mite infestation (Otodectes cynotis)
- Treatment and control of intestinal worms
Roundworm (Toxocara cati)
Hookworm (Ancylostoma tubaeforme)What should I discuss with my veterinarian before SelaSpot is prescribed?
Your veterinarian is best suited to discuss and recommend appropriate medications for your dog or cat. It is important to discuss your pet's health history with your veterinarian so he/she can decide if SelaSpot is right for your animal.SelaSpot should not be used in sick, debilitated or underweight animals.
Dogs should be tested for heartworm disease prior to giving SelaSpot. If your dog tests positive for adult heartworms, your veterinarian can recommend appropriate treatment. Dogs infected with adult heartworms can safely be given SelaSpot.
If your cat is older than six months of age, your veterinarian may decide to test him/her for heartworm disease before prescribing SelaSpot. Cats infected with adult heartworms can be given SelaSpot to prevent further infections.What dose of SelaSpot do I use on my dog or cat?
Your veterinarian will recommend the appropriate dose for your dog or cat based on your animal's body weight. You should not administer SelaSpot to dogs younger than 6 weeks of age or cats younger than 8 weeks of age. SelaSpot is available in eight separate dose strengths for dogs and cats of different weights.What should I do if I do not give SelaSpot on time or miss a dose?
If you forget to apply a monthly dose of SelaSpot immediately apply SelaSpot, resume monthly applications, and notify your veterinarian.What if I administer more than the prescribed amount of SelaSpot to my dog or cat?
Contact your veterinarian if you administer more than the prescribed amount of SelaSpot.How should SelaSpot be applied?
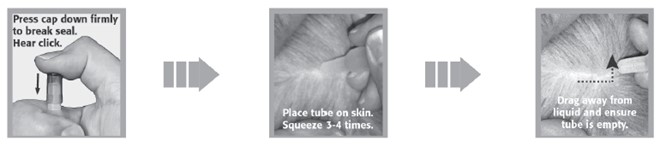
1. Remove the SelaSpot tube from its protective package.
2. Holding the tube upright, firmly press the cap down until you hear a 'click' sound, which signals that the cap has punctured the applicator seal. Remove the cap and check to be sure that the tip of the tube is open.
3. Part the hair on the back of the animal at the base of the neck, in front of the shoulder blades, until the skin is visible.
4. Apply the tip of the SelaSpot tube directly to the skin. Squeeze the tube firmly 3–4 times in one spot until empty. Keep tube compressed on the final squeeze to avoid drawing liquid back into tube. Avoid contact between SelaSpot and your fingers.
5. While keeping tube squeezed, drag it away from liquid and lift up to remove.
6. Ensure tube is empty.
Do not massage SelaSpot into the skin.
Do not apply when the haircoat is wet.
Do not apply to broken skin – SelaSpot contains alcohol.Stiff hair, clumping of hair, hair discoloration, or a slight powdery residue may be observed at the site in some animals. These effects are usually temporary and do not affect the safety or effectiveness of the product.
Can I give my pet a bath after applying SelaSpot?
Yes. Bathing or shampooing the dog 2 or more hours after treatment will not reduce the effectiveness of SelaSpot against fleas or heartworm. Bathing or shampooing the cat 24 hours after treatment will not reduce the effectiveness of SelaSpot against fleas or heartworm.When can I play with my pet following treatment with SelaSpot?
You should avoid contact with application site when wet. You may hold or play with your pet any time after the area on which SelaSpot was applied is dry.I see fleas on my dog or cat. Is SelaSpot working?
SelaSpot kills adult fleas and prevents flea eggs from hatching. You may occasionally see a few fleas on dogs or cats treated with SelaSpot but more than 98% of adult fleas are killed within 36 hours.
Immature stages of the flea called pupae may be present in your pets' environment (yard, flooring, carpet, bedding, etc.). These pupae are not killed by parasiticides (including SelaSpot) and as such may emerge as adult fleas. These adult fleas may hop onto your pet at anytime. They must be exposed to SelaSpot on your dog or cat before being killed. It can take from 3–5 weeks (or longer depending on environmental conditions) for most fleas to complete their 4-stage life cycle (egg, larvae, pupae, and adult) and reach the adult stage before being seen on your pet. Due to the presence of immature flea stages in infested environments it can take up to 2 to 3 monthly applications for SelaSpot to maximally control the infestation of fleas in the environment. Once the flea population is controlled you will be less likely to see fleas.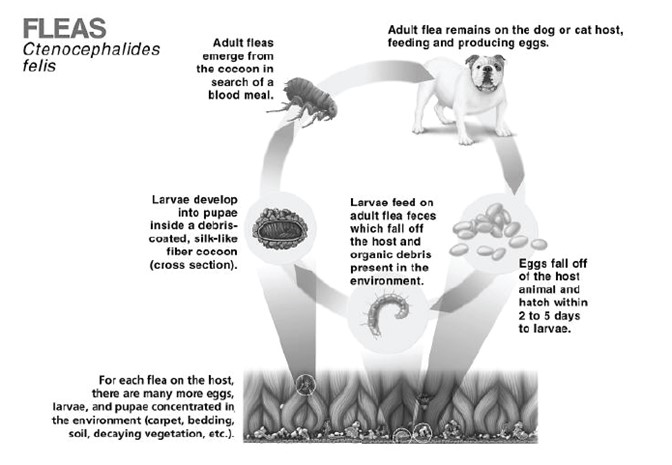
I see ticks on my dog. Is SelaSpot working?
SelaSpot controls tick infestations only due to the American Dog Tick (Dermacentor variabilis), a tick commonly found on dogs. There are other common species of ticks that are not killed or controlled by SelaSpot. Your veterinarian can recommend appropriate products to control or kill ticks common to your area. For the control of the American Dog Tick, SelaSpot should be applied once a month; however, your veterinarian may recommend a second administration applied 14 days after the first dose if your dog has a heavy tick infestation and/or recommend additional tick control methods. It may take up to 5 days to kill the majority of ticks on your dog.
What are the possible side effects of SelaSpot?
Following the use of selamectin, the following side effects have been seen, listed in decreasing order of frequency for each species.
Dogs- Sluggishness, vomiting, diarrhea (with or without blood), decreased appetite, generalized itching, seizures, hair loss or skin redness at the application site, trembling, incoordination, skin inflammation, drooling, and rapid breathing.
In some cases, death has been reported in dogs.
Cats- Hair loss at the site of application with or without redness, flaking, or itching, sluggishness, decreased appetite, vomiting, generalized itching, diarrhea (with or without blood), incoordination, fever, hair loss, trembling, drooling, skin inflammation, and seizures.
Severe application site reactions like blisters, scabbing, and infection have been reported in cats.
In some cases, death has been reported in cats.Can SelaSpot be given with other medicines?
In well-controlled clinical studies, selamectin was used safely in dogs and cats receiving other veterinary products such as vaccines, anthelmintics, antiparasitics, antibiotics, steroids, collars, shampoos and dips.
Tell your veterinarian about all medicines you have given your dog or cat in the past, and any medicines that you are planning to use with SelaSpot. This should include other medicines that you can get without a prescription. Your veterinarian may want to check that all of your dog's or cat's medicines can be given together.How should SelaSpot be stored?
SelaSpot is flammable – Keep away from heat, sparks, open flames or other sources of ignition. Store below 25°C (77°F). After application, empty tubes can be placed in your normal household refuse for disposal.What else should I know about SelaSpot?
SelaSpot is not for use in humans.
SelaSpot should be kept out of reach of children.
In humans, SelaSpot may be irritating to skin and eyes. Reactions such as hives, itching and skin redness have been reported in humans. Individuals with known hypersensitivity to SelaSpot should use the product with caution or consult a health care professional.
SelaSpot contains isopropyl alcohol and the preservative butylated hydroxytoluene (BHT).
Wash hands after use and wash off any product in contact with skin immediately with soap and water. In case of human ingestion contact a doctor immediately.Approved by FDA under ANADA # 200-696
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-317 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-317-99 3 in 1 CARTON 1 0.25 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-318 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-318-99 3 in 1 CARTON 1 0.75 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-319 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-319-99 3 in 1 CARTON 1 1 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-320 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-320-99 3 in 1 CARTON 1 0.25 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-321 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-321-99 3 in 1 CARTON 1 0.5 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-322 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-322-99 3 in 1 CARTON 1 1 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-323-99 3 in 1 CARTON 1 2 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 SELASPOT
selamectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50989-324 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELAMECTIN (UNII: A2669OWX9N) (SELAMECTIN - UNII:A2669OWX9N) SELAMECTIN 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50989-324-99 3 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200696 12/10/2025 Labeler - Vedco, Inc. (021634266) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture
Trademark Results [SelaSpot]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SELASPOT 88830555 not registered Live/Pending |
BIMEDA Animal Health Limited 2020-03-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.