VIONEXUS- alcohol liquid
VIONEXUS by
Drug Labeling and Warnings
VIONEXUS by is a Otc medication manufactured, distributed, or labeled by METREX RESEARCH, LLC, METREX RESEARCH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Warnings
- Uses
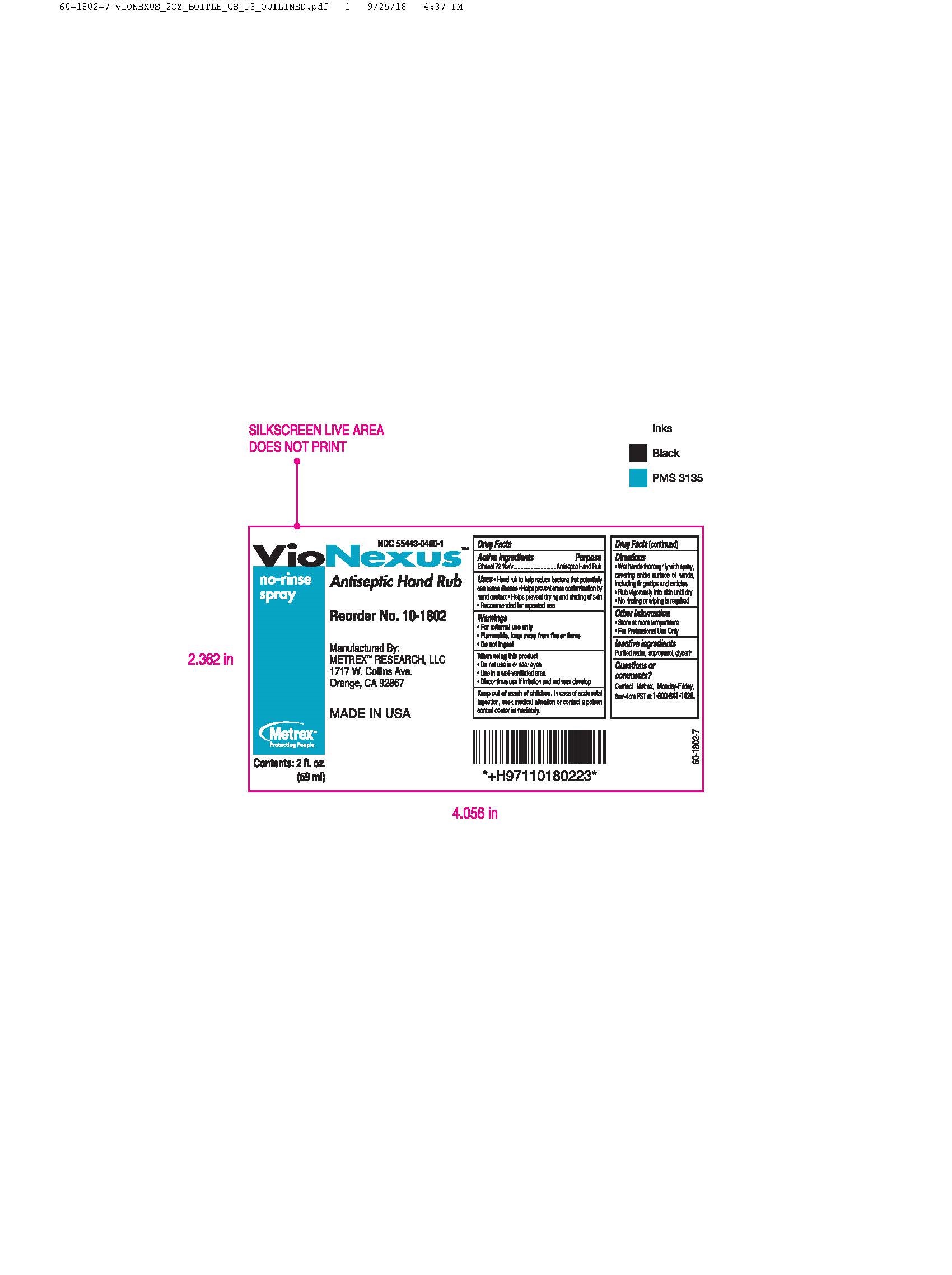
- Active ingredients
- Directions
- Other information
- Inactive ingredients
-
Questions or comments?
For product or technical information, contact Metrex, Monday to Friday, 6am - 4pm PST at 800-841-1428 or visit our website at www.metrex.com.
- Dosage and Administration Route(s)
- Vionexus No Rinse Spray Label
-
INGREDIENTS AND APPEARANCE
VIONEXUS
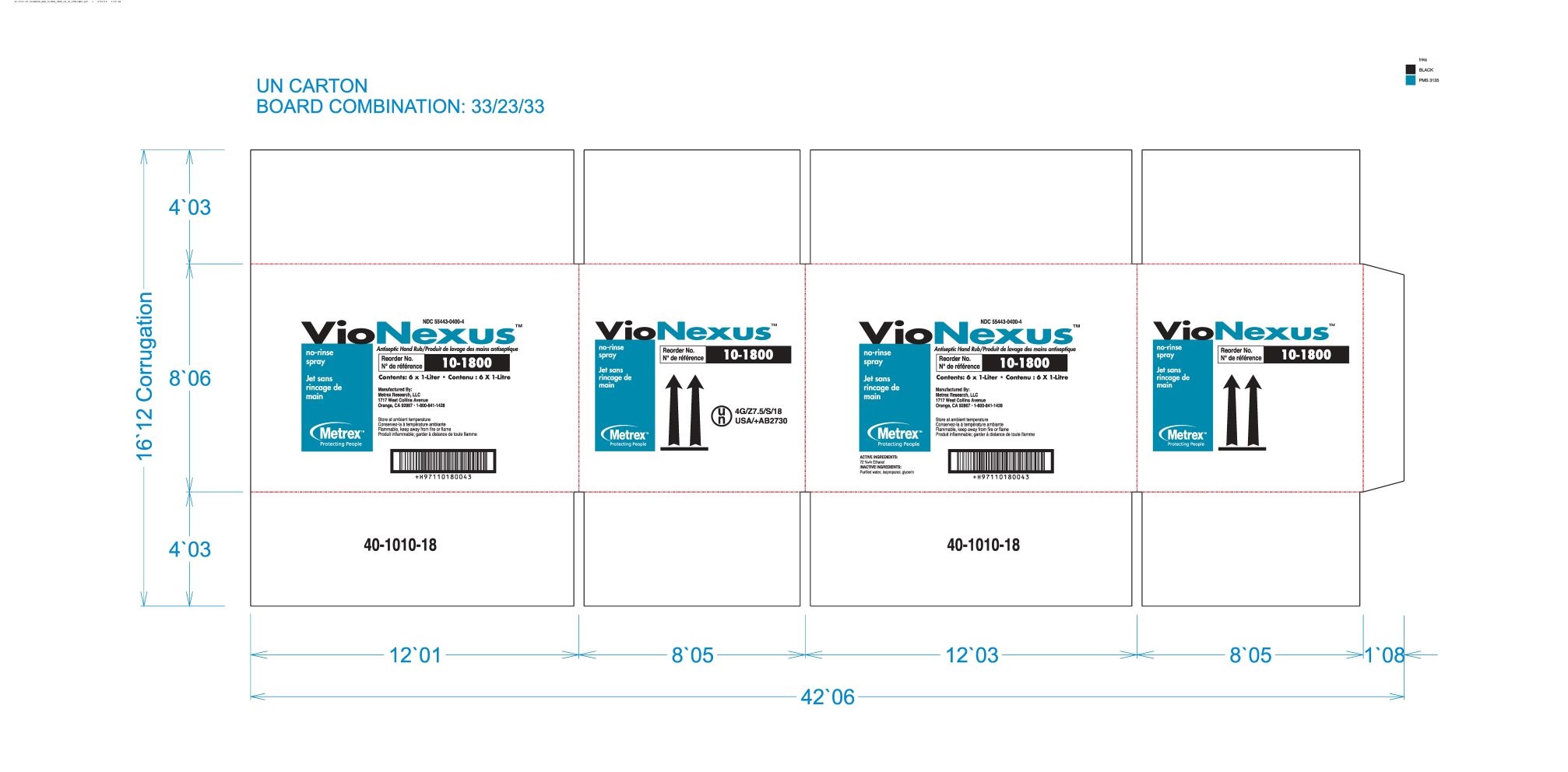
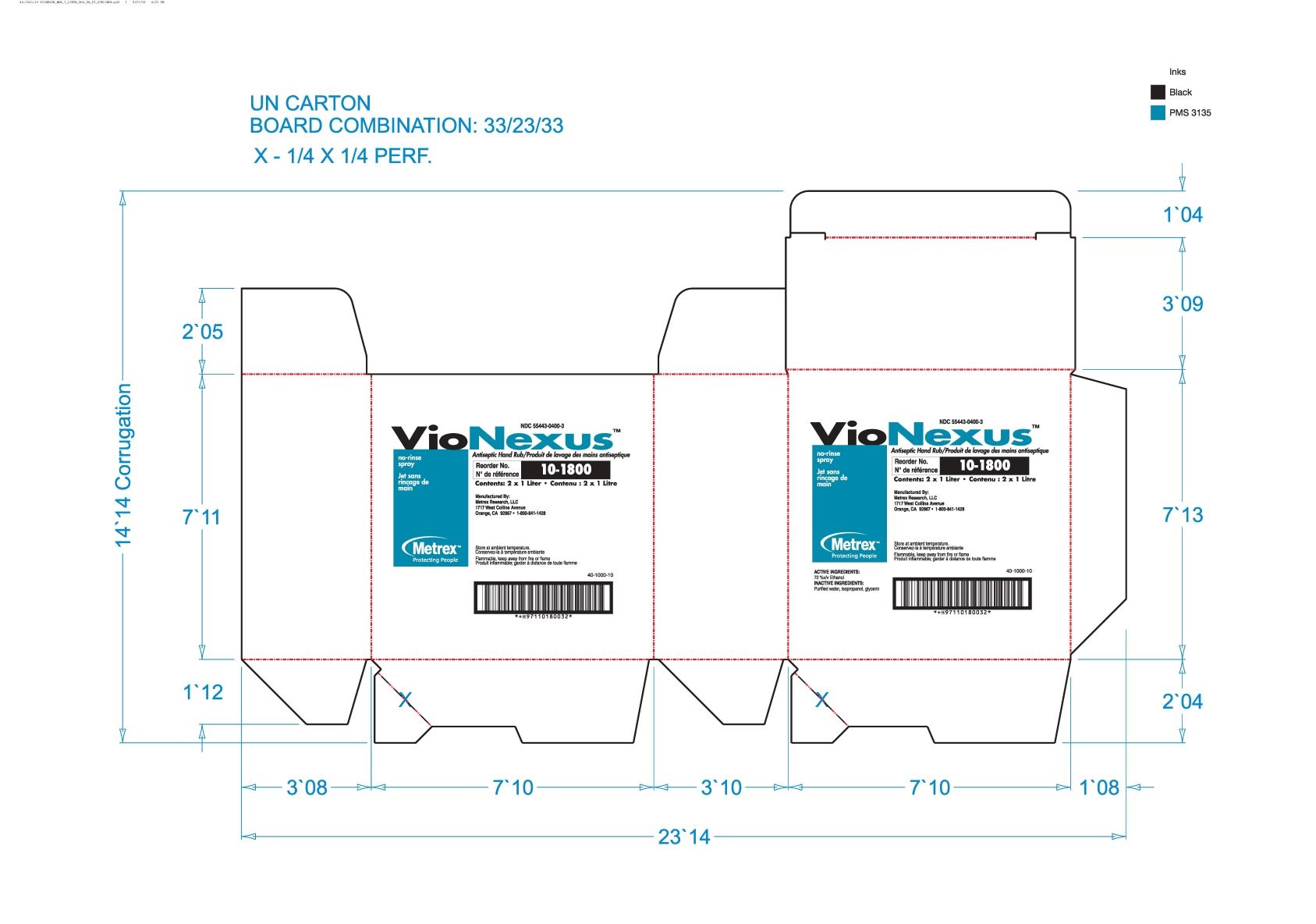
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55443-0400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 72 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL ALCOHOL (UNII: ND2M416302) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55443-0400-5 48 in 1 CASE 11/01/2018 1 NDC: 55443-0400-1 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC: 55443-0400-4 3 in 1 CASE 11/01/2018 2 NDC: 55443-0400-3 2 in 1 BOX 2 NDC: 55443-0400-2 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 3 NDC: 55443-0400-7 12 in 1 CASE 04/01/2020 3 NDC: 55443-0400-6 237 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 11/01/2018 Labeler - METREX RESEARCH, LLC (102567567) Registrant - METREX RESEARCH (102567567) Establishment Name Address ID/FEI Business Operations Metrex Research 102567567 label(55443-0400) Establishment Name Address ID/FEI Business Operations METREX RESEARCH 145963778 manufacture(55443-0400)
Trademark Results [VIONEXUS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIONEXUS 85697923 4316679 Live/Registered |
Metrex Research, LLC 2012-08-08 |
 VIONEXUS 85494011 not registered Dead/Abandoned |
Metrex Research, LLC 2011-12-13 |
 VIONEXUS 76127921 not registered Dead/Abandoned |
Metrex Research Corporation 2000-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.